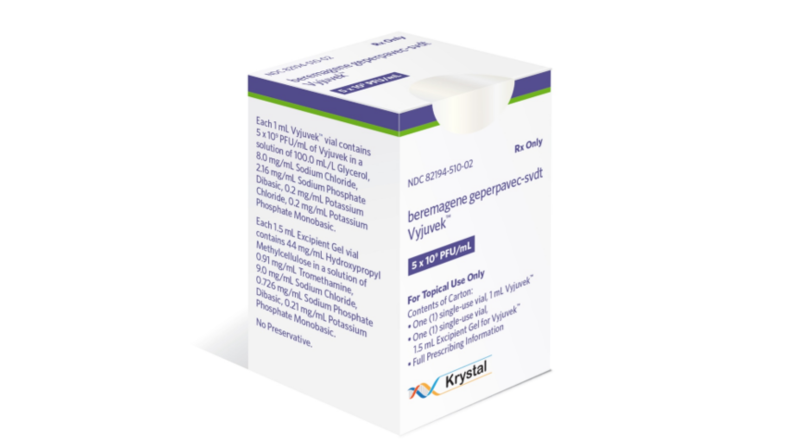
FDA Approves Krystal Biotech’s VYJUVEK™: First Redosable Skin-disorder Gene Therapy
Krystal Biotech, Inc., a Pennsylvania-headquartered biotech company specialising in genetic medicines for rare diseases, announced on Friday that the US FDA has approved VYJUVEK™ (beremagene geperpavec-svdt) for the treatment of Dystrophic Epidermolysis Bullosa (DEB) in patients aged six months and above.
The first-of-its-kind gene therapy for Dystrophic Epidermolysis Bullosa
VYJUVEK™ is a groundbreaking redosable gene therapy, in the form of a topical gel, that targets the genetic cause of DEB by delivering functional copies of the human COL7A1 gene. It promotes wound healing and sustains the expression of functional COL7 protein. The freshly FDA-approved therapy is the first of its kind for DEB, and it can be administered by healthcare professionals in various settings, including the patients’ homes.
DEB is a rare and severe condition affecting the skin and mucosal tissues. It arises from one or more mutations in the COL7A1 gene, which is responsible for producing functional COL7 protein. This protein plays a crucial role in forming anchoring fibrils that bind the inner layer of the skin (dermis) to the outer layer (epidermis). In individuals with DEB, the absence of functional anchoring fibrils results in highly fragile skin that easily blisters and tears even with minor friction or injury. Patients with DEB experience chronic open wounds, which frequently lead to skin infections and fibrosis. This can cause fusion of fingers and toes and significantly raises the risk of developing aggressive forms of skin cancer.
M. Peter Marinkovich, M.D., primary investigator of the GEM-3 trial, Director of the Blistering Disease Clinic at Stanford Health Care and Associate Professor of Dermatology at the Stanford University School of Medicine, said:
“This is a devastating disease. Until now, doctors and nurses had no way to stop blisters and wounds from developing on dystrophic EB patient skin and all we could do was to give them bandages and helplessly watch as new blisters formed. VYJUVEK topical gene therapy changes all of this. VYJUVEK both heals patient wounds and prevents skin from re-blistering because it actually corrects the underlying skin defect of dystrophic EB. Because it’s safe and easy to apply directly to wounds, it doesn’t require a lot of supporting technology or specialized expertise, making VYJUVEK highly accessible even to patients who live far away from specialized centers.”
Phase 3 trial met its primary endpoint of complete wound healing
The approval of VYJUVEK™ by the FDA was supported by two clinical studies. The GEM-1/2 trial, a single-center study, demonstrated that repeated topical applications of VYJUVEK resulted in lasting wound closure, complete expression of cutaneous COL7 protein, and the formation of anchoring fibrils. Minimal adverse events were reported in this intra-patient, open-label, randomized, placebo-controlled trial. In the GEM-3 trial, a multi-center study, VYJUVEK™ achieved its primary endpoint of complete wound healing at six months and its key secondary endpoint of complete wound healing at three months in a double-blinded, randomized, placebo-controlled design. The therapy was well tolerated, with no serious adverse events related to the drug or treatment discontinuations.
Suma Krishnan, President, Research & Development, Krystal Biotech, Inc., said:
“Data from our GEM-1/2 trial and our GEM-3 trial, published in Nature Medicine and the New England Journal of Medicine, respectively, demonstrated the strength of both studies showing that VYJUVEK safely and effectively improved wound healing. For so many years, all we have been able to offer DEB patients was palliative care, but now, based on the strength of the Company’s clinical trial data, there is a safe and effective FDA approved treatment.”
Krish S. Krishnan, Chairman and Chief Executive Officer of Krystal Biotech, Inc. stated:
“Today’s landmark approval of VYJUVEK as the first redosable gene therapy ushers in a whole new paradigm to treat genetic diseases and is an important milestone for patients affected by DEB as well as their families and caregivers. We offer our sincere gratitude to DEB patients, caregivers, investigators, US regulators, and our employees who made this approval possible. For Krystal, this is a transformative achievement that highlights our commitment to developing and commercializing novel therapies for patients with rare diseases and demonstrates Krystal’s capability as a fully-integrated company ready to launch and bring VYJUVEK to patients as quickly as possible and deliver additional transformative medicines to patients as we advance our pipeline.”
VYJUVEK™ is anticipated to be accessible in the United States by the third quarter of 2023, and the Company will promptly commence its promotion. To provide comprehensive support to patients, caregivers, and families throughout their VYJUVEK™ treatment journey, the Company has established Krystal Connect, a personalized support program. This program offers various resources, including answers to inquiries about VYJUVEK, verification of health benefits, assistance with treatment planning and administration, and information on financial aid options for eligible patients. For further details, patients, caregivers, and healthcare professionals can contact Krystal Connect at 1-844-5-KRYSTAL.
Following the approval, the FDA awarded the Company a Rare Pediatric Disease Priority Review Voucher (PRV), granting priority review to a future drug application that may not otherwise qualify for such consideration. The PRV program aims to incentivize the development of novel drugs for the prevention or treatment of rare diseases.
Brett Kopelan, Executive Director of debra of America, the national organization dedicated to improving the lives of all people living with EB in the US. added:
“With the FDA approval of VYJUVEK, the DEB population has reached a monumental milestone in the treatment of this horrible disorder. Our hopes have now been realized for a safe and effective treatment for one of the most devastating symptoms of the disorder. We thank Krystal for their dedication and commitment to bringing VYJUVEK to fruition. People living with DEB will now have a significant chance of having an improved quality of life and debra will continue to work closely with Krystal to assure patients have ready access to VYJUVEK.”
In regions beyond the United States, the European Medicines Agency (EMA) has bestowed VYJUVEK with orphan drug designation and eligibility for PRIME (PRIority MEdicines) for the treatment of DEB. The Company foresees commencing the official Marketing Authorization Application procedure in the latter half of 2023, with the possibility of obtaining approval in 2024. Additionally, the Company is collaborating with the Pharmaceuticals and Medical Devices Agency in Japan to conduct studies on VYJUVEK™ and pursue approval for a possible launch in 2025.
Please visit VYJUVEK.com for more information.
Original Source: Press Release, May 19, 2023, Krystal Biotech, Inc.: https://ir.krystalbio.com/news-releases/news-release-details/krystal-biotech-receives-fda-approval-first-ever-redosable-gene
Image Credits: Krystal Biotech, Inc.
Recommended Companies
More Headlines