Boosting Retinal Metabolism to Preserve Cone Function
A recent study from Constance Cepko’s group at Harvard Medical School offers an encouraging advance toward therapeutic strategies for Retinitis Pigmentosa, a progressive retinal disorder that affects roughly 1 in 4000 individuals globally. Retinitis Pigmentosa can arise from mutations in more than a hundred different genes. These mutations primarily impact rod photoreceptors, ultimately causing their degeneration, which explains why early symptoms frequently include compromised night vision due to rod loss. At more advanced stages, cone photoreceptors, essential for daylight vision and colour discrimination, also die. In contrast to rods, many of the cone-associated defects appear to result from metabolic imbalance rather than direct genetic impairment.
The Cepko laboratory focuses on determining whether reinforcing retinal metabolic pathways in Retinitis Pigmentosa can slow cone deterioration and maintain visual function, independent of the specific mutation that triggers rod degeneration. A strategy like this could benefit a wide range of Retinitis Pigmentosa patients, unlike classic gene therapy approaches that typically target only one causative mutation. In this work, Cepko and colleagues examined whether improving retinal pigment epithelium (RPE) metabolism could postpone cone degeneration. Using an AAV vector to elevate expression of the lactate transporter MCT2 in the RPE, the researchers sought to enhance lactate uptake from the choroidal circulation, thereby sparing more glucose to support cone photoreceptors. Their findings demonstrated not only improved cone preservation and performance in animal models, but also unveiled a new method for directly imaging glucose and lactate concentrations within RPE cells.

PUBLICATION
Proceedings of the National Academy of Sciences of the United States of America (Apr 08, 2025) ”RPE-specific MCT2 expression promotes cone survival in models of retinitis pigmentosa”
Chandler LC, Gardner A, Cepko CL
The Central Importance of RPE Metabolic Function
Retinitis Pigmentosa is initiated by the degeneration of rod photoreceptors, yet cone loss typically follows, prompting the question of why cones eventually die despite the fact that, in most forms of Retinitis Pigmentosa, they do not harbour the disease-causing mutation. A key explanation lies in their distinctive metabolic requirements. Because the photoreceptor layer lacks its own blood supply, both rods and cones depend on the underlying retinal pigment epithelium (RPE) for nutrient support. Photoreceptors consume substantial amounts of glucose, processing it through aerobic glycolysis and generating lactate as a metabolic byproduct. Under normal conditions, this lactate is absorbed by the RPE via MCT1/2 transporters. Within RPE cells, part of the lactate is used metabolically, while the remainder is returned to circulation. Critically, this lactate uptake suppresses glycolysis within the RPE, helping ensure that glucose remains accessible to photoreceptors.
When rods degenerate, this finely tuned metabolic feedback system collapses. As lactate delivery to the RPE decreases, the RPE reactivates glycolysis to satisfy its own energetic demands, thereby consuming glucose that would otherwise support cone photoreceptors. The outcome is a glucose deficit for cones and progressive vision deterioration, a self-reinforcing metabolic decline. Cepko’s team proposed that enhancing lactate uptake in RPE cells could re-establish metabolic balance and safeguard cones from degeneration.
AAV-Based Enhancement of MCT2 Expression
To evaluate this metabolic intervention, the researchers designed an AAV vector encoding the high-affinity monocarboxylate transporter MCT2 under control of an RPE-specific promoter. Because MCT2 exhibits stronger lactate import capacity than MCT1 or MCT3, its overexpression was anticipated to compensate for the decline in rod-derived lactate. The vector was administered via subretinal injection in both rat and mouse models of Retinitis Pigmentosa, allowing direct comparison of cone preservation and performance between treated and untreated retinal regions.
Cone Maintenance in Retinal Degeneration Models
In rats harbouring a pathogenic mutation, neonatal delivery of the MCT2 vector produced measurable protective effects. Six months post the injection, cone quantification demonstrated that treated retinas retained more cones than non-transduced controls, both across entire retinal fields and within areas receiving vector expression. Although the degree of preservation fell short of that seen in healthy animals, it was nevertheless superior to untreated conditions.
Similarly, in two mouse strains (FVB and P23H) that characteristically exhibit “cone crater” degeneration patterns, MCT2 supplementation again yielded improved outcomes. Cone density was higher, and the typical crater-like lesions were absent in treated retinas. To precisely map regions of transduction, an additional AAV expressing GFP in cone nuclei was used, confirming broad cone-sparing effects. As with the rat experiments, the rescue was substantial but incomplete.
Sustained Cone Function in Treated Eyes
Structural preservation alone does not guarantee functional benefit, so the investigators next evaluated whether cones retained visual capacity. Using Striatech’s OptoDrum to measure the optomotor response in P23H mice, they observed enhanced visual acuity in animals receiving MCT2 compared to untreated controls. Variability arose from differences in injection coverage and natural biological variation, but the overall trend favoured improved performance. Notably, these gains diminished over time, and by postnatal day 53, treated and control groups were no longer statistically distinguishable. These findings indicate that MCT2 augmentation extends but does not indefinitely maintain cone-mediated vision.

Reproduced from Figure 2 of the original publication under the Creative Commons Attribution (CC BY 4.0) license.
Direct Assessment of Metabolic Shifts Using FLIM-Based Biosensors
To verify that the improved cone preservation stemmed from altered metabolic activity, the team utilised an advanced strategy involving fluorescence lifetime imaging (FLIM) biosensors. These genetically encoded probes, delivered via AAV vector, change their conformation upon binding specific metabolites such as lactate (LiLac sensor) or glucose (GlucoSnFR-TS), producing measurable shifts in fluorescence lifetime. When paired with two-photon microscopy, this system enabled direct, single-cell–resolution measurements of metabolite levels within living RPE tissue ex vivo.
In eyecups transduced with MCT2, lactate biosensors detected elevated intracellular lactate at baseline and enhanced uptake across physiological concentration ranges relative to controls. Glucose sensors similarly revealed higher intracellular glucose in treated RPE under increased medium glucose levels, consistent with reduced glycolytic utilisation by the RPE. These metabolic readouts provided direct confirmation of the study’s overarching hypothesis.
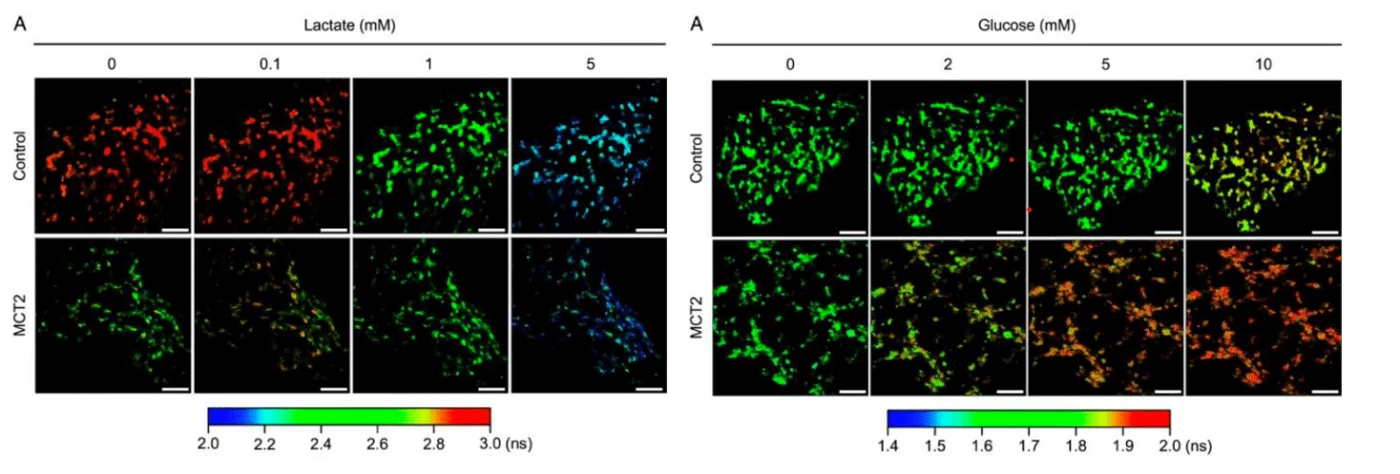
Reproduced from Figure 4A (Lactate) and 5A (Glucose) of the original publication under the Creative Commons Attribution (CC BY 4.0) license.
Potential and Limitations of Metabolic Rescue in Retinitis Pigmentosa
Taken together, these research studies indicate that increasing MCT2-driven lactate uptake in the RPE can partially sustain cone survival and extend visual function in Retinitis Pigmentosa models. The use of FLIM biosensors introduces a powerful approach for probing retinal metabolism, offering single-cell resolution and overcoming several constraints of traditional techniques.
However, important challenges remain. Even with MCT2 overexpression, cones ultimately degenerated, suggesting that additional contributors, such as inflammation or oxidative stress, continue to drive disease progression. In addition, the ex vivo metabolic assays, although sophisticated, may not fully reproduce in vivo physiology. Future research may refine these approaches, including the development of live in vivo FLIM imaging, and test combination strategies that target multiple pathological pathways.
Overall, this work supports the potential of gene-agnostic interventions that correct metabolic dysfunction as a shared mechanism of cone loss in Retinitis Pigmentosa. By extending central vision independent of the specific mutation, such therapies could provide broad clinical benefit for patients facing this currently incurable disease.
Original Source: Emilia Kawecka, Technical University of Munich, Student Assistant at Striatech
Original Paper: L.C. Chandler, A. Gardner, & C.L. Cepko, RPE-specific MCT2 expression promotes cone survival in models of retinitis pigmentosa, Proc. Natl. Acad. Sci. U.S.A. 122 (14) e2421978122, https://doi.org/10.1073/pnas.2421978122 (2025).
Related Product