FEATURED
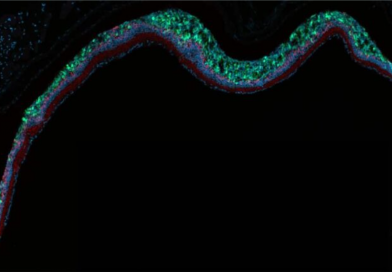
Journal Club: Photoreceptor Cell Therapy to Treat Advanced Retinal Degeneration
Striatech is pleased to announce its upcoming Journal Club session, “Photoreceptor Cell Therapy to Treat Advanced Retinal Degeneration,” which
DEALS
Sanofi Strengthens Vaccine Portfolio with $2.2B Dynavax Acquisition
Sanofi has announced it has agreed to acquire Dynavax Technologies Corporation, a publicly traded vaccines company, in a move that
PRESS RELEASES

Journal Club: Photoreceptor Cell Therapy to Treat Advanced Retinal Degeneration
Striatech is pleased to announce its upcoming Journal Club session, “Photoreceptor Cell Therapy to Treat Advanced Retinal Degeneration,” which
EDITOR’S PICKS

Alpha-Synuclein Aggregation: A Key Target in the Battle Against Parkinson’s Disease
Parkinson’s disease (PD) is a progressive neurodegenerative condition impacting millions globally. While a blend of genetic, environmental, and ageing factors
RESOURCES

A Game-Changer in Predicting Drug-Induced Gastrointestinal Toxicity
CacoReady, a Useful Cell Model to Predict Drug-Induced Gastrointestinal Toxicity A ReadyCell .pdf Case Study – Author: Marta Olle Monge,
MANUFACTURING PROJECTS

Eli Lilly to Invest $6 Billion in New API Manufacturing Facility in Alabama, USA
The new Huntsville, Alabama site will produce small molecule and peptide medicines, including oral GLP-1 orforglipron Eli Lilly and Company
CLINICAL TRIALS

Lilly’s Jaypirca Outperforms Imbruvica in CLL Head-to-Head Phase 3 Trial
Eli Lilly’s Jaypirca (pirtobrutinib), the first and only FDA-approved non-covalent (reversible) BTK inhibitor, has met its primary endpoint in the
REGULATORY

FDA Approves Novo’s Wegovy® Pill as First Oral GLP-1 Weight Loss Therapy
Novo Nordisk A/S has announced that the US Food and Drug Administration (FDA) has approved the Wegovy® pill (once-daily oral
SELECTED PRODUCTS

In Vivo Assessment of Visual Performance in Mice
The unique OptoDrum device for automated in vivo assessment of visual performance in mice and rats offers unmatched capabilities for