Alpha-Synuclein Aggregation: A Key Target in the Battle Against Parkinson’s Disease
Parkinson’s disease (PD) is a progressive neurodegenerative condition impacting millions globally. While a blend of genetic, environmental, and ageing factors may contribute to its progression, the exact etiology remains elusive. Presently, there is no cure, with only limited treatments offering relief from motor symptoms. Researchers are actively exploring potential causative factors and advancing therapeutic avenues.
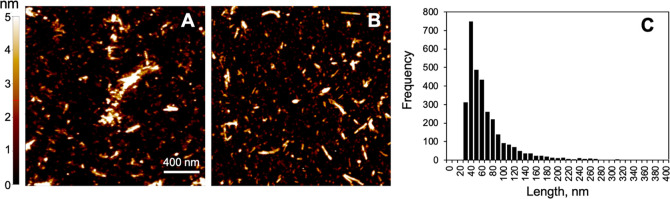
Central to PD pathogenesis is the role of alpha synuclein, whose abnormal accumulation and aggregation give rise to Lewy bodies and Lewy neurites, characteristic markers of the disease. Both oligomeric and fibrillary forms of alpha synuclein exhibit neurotoxicity and propagate in a prion-like manner, spreading across various brain regions. Numerous investigations emphasize the importance of targeting these aggregates in developing novel therapeutic approaches. Through incubation, recombinant alpha synuclein monomers assemble into pre-formed fibrils (PFFs), providing a model for PD. Introduction of these PFFs into cells or animals can induce aggregation of endogenous alpha synuclein and Lewy body pathology.
Alpha synuclein aggregates engage with membranes of neuronal cells
Numerous studies of Alpha synuclein-membrane interactions have shown that toxic oligomeric and fibrillary forms of the protein can lead to rapid destruction of the membranes. Particularly, different aggregates can alter membrane morphology and permeability. These interactions seem to impact both protein and membrane properties as they lead to membrane disruption and affect the kinetics of alpha synuclein fibril formation.
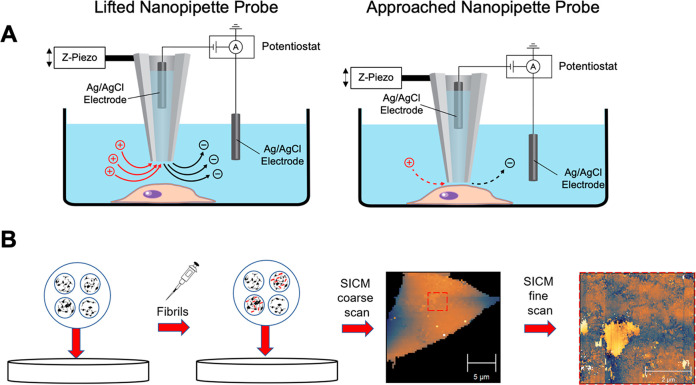
Scientists and researchers from California State University in LA utilized alpha synuclein oligomers and PFFs to examine their impact on neuronal cell membranes and proposed potential strategies for preventing neuronal damage in PD patients. In their initial investigation, they exposed live SH-SY5Y neuroblastoma cells to alpha synuclein oligomers and employed Scanning Ion Conductance Microscopy (SICM) to observe changes in membrane morphology. Elevated concentrations of alpha synuclein oligomers disrupted the lipid bilayer by creating transient pores on the membrane, ultimately resulting in membrane disruption. In a subsequent study published in the ACS Chemical Neuroscience journal, titled “Observation of α-Synuclein Preformed Fibrils Interacting with SH-SY5Y Neuroblastoma Cell Membranes Using Scanning Ion Conductance Microscopy“, the researchers employed StressMarq’s human alpha synuclein PFFs (catalog# SPR-317) to explore the effects of fibril exposure on SH-SY5Y cell membranes.
The impact of alpha synuclein PFFs on neuroblastoma cell membranes
Christina Feng and her team explored how exposing SH-SY5Y neuroblastoma cell membranes to alpha synuclein fibrils affects them, utilizing Scanning Ion Conductance Microscopy (SICM). They introduced various concentrations of alpha synuclein PFFs to neuroblastoma cells over a 48-hour period. Subsequently, they examined the cell membranes using SICM, a method capable of providing high-resolution, non-contact imaging of living tissue and cell surfaces in real-time. The SICM images revealed a notable rise in membrane roughness with increasing fibril concentration.
The researchers also evaluated cell viability and cytotoxicity to provide additional insights into the SICM images. The XTT viability assay revealed a slight yet statistically significant decline in cell viability, while the LDH cytotoxicity assay showed only a marginal increase in LDH release, both regardless of fibril concentration. These assay outcomes imply that PFFs might impact membranes and interfere with cellular function without inducing cell demise. The study’s results suggest that addressing pathological alpha synuclein fibrils prior to their accumulation and subsequent aggregation could potentially mitigate neuron loss during the early stages of PD, offering a promising avenue for therapeutic development.
StressMarq’s fibrillary proteins support Parkinson’s disease research
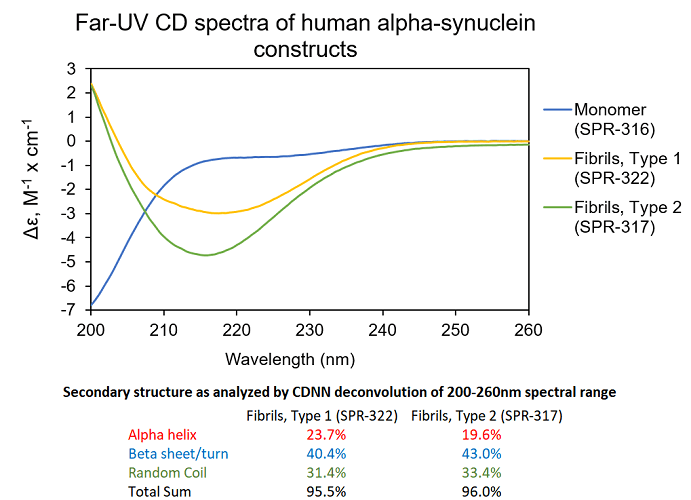
StressMarq provides a broad variety of Alpha Synuclein Monomers, Oligomers and Pre-formed fibrils, useful for modelling Parkinson’s disease because of the company’s ability to induce aggregation and pathology in different cellular and animal models. These include Type 2 Human Alpha Synuclein Pre-formed Fibrils (catalog# SPR-317) which have been shown to interact with neuronal membranes without causing cell death.
In addition to alpha synuclein fibrillar proteins, StressMarq offers a range of antibodies, proteins, kits, and small molecules for Parkinson’s disease research, including Amyloid Beta and Tau.
Related Products