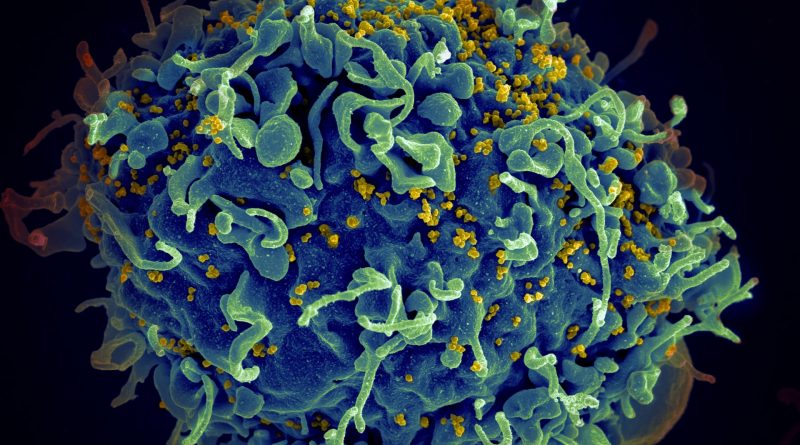
Moderna Starts HIV Vaccine Trial that Uses Breakthrough mRNA Technology
The first doses of an experimental HIV vaccine antigen have been provided in a clinical study at George Washington University (GWU) School of Medicine and Health Sciences in Washington, D.C., according to IAVI, a nonprofit scientific research organization, and Moderna, a pharmaceutical and biotechnology company.
Phase 1 trial to use Moderna’s mRNA technology to deliver immunogens created by IAVI and Scripps Research scientists
IAVI G002 is a Phase 1 study that aims to see if consecutive administration of priming and boosting HIV immunogens using messenger RNA (mRNA) might stimulate certain types of B-cell responses and steer their early maturation toward the synthesis of broadly neutralizing antibodies (bnAbs). The induction of bnAbs is usually seen as a goal of HIV vaccine, and this is the first step. IAVI G002 plans to use Moderna’s mRNA technology to deliver immunogens created by IAVI and Scripps Research scientists.
Mark Feinberg, M.D., Ph.D., president and CEO of IAVI said:
“We are tremendously excited to be advancing this new direction in HIV vaccine design with Moderna’s mRNA platform. The search for an HIV vaccine has been long and challenging, and having new tools in terms of immunogens and platforms could be the key to making rapid progress toward an urgently needed, effective HIV vaccine. We are grateful to all of our partners and especially to the Bill & Melinda Gates Foundation for funding this trial.”
Stephen Hoge, M.D., President of Moderna stated:
“We are very pleased to be partnering with IAVI and the Bill & Melinda Gates Foundation to apply our mRNA technology in the setting of HIV. At Moderna, we believe that mRNA offers a unique opportunity to address critical unmet public health needs around the world. We believe advancing this HIV vaccine program in partnership with IAVI and Scripps Research is an important step in our mission to deliver on the potential for mRNA to improve human health.”
William Schief, Ph.D., professor at Scripps Research and executive director of vaccine design at IAVI’s Neutralizing Antibody Center (NAC), and colleagues engineered the HIV vaccine antigens in this study as proteins. Dr. Schief released findings from the IAVI G001 clinical study in 2021, revealing that an adjuvanted protein-based variant of the priming immunogen (eOD-GT8 60mer) elicited the required B-cell response in 97% of participants. IAVI G002 not only examines the efficacy of a boosting immunogen to generate additional maturation of B cells, but also tests priming of the desired immunological response utilizing eOD-GT8 60mer mRNA delivery. This technology offers a more agile and responsive approach to vaccine development due to the rapidity with which mRNA vaccines may be manufactured.
The Schief lab was one of the first to use the revolutionary vaccine development platform known as germline targeting Unmutated, or “germline,” genes that encode antibodies in naive B cells. A series of vaccinations, beginning with the prime-boost immunogens used here, might target certain naïve B cells and encourage them to develop into bnAb-producing cells. bnAbs have been proven in the lab to neutralize a wide range of HIV variations, and one of them, VRC01, has recently been shown to protect people from infection by neutralization-susceptible HIV strains. VRC01 belongs to the bnAb class that IAVI G001 is looking for.
William Schief, Ph.D., professor at Scripps Research and executive director of vaccine design at IAVI’s Neutralizing Antibody Center (NAC) stated:
“We’ve seen promising proof of concept for germline targeting in IAVI G001, and this trial lets us take that approach to the next stage. What’s more, we’ve been able to expedite production of clinical trial material at a remarkably rapid pace because of Moderna’s technology.”
The discovery of these vaccine antigens was made possible through years of collaboration between IAVI and Scripps Research as part of a long-standing NAC alliance. As they broaden and assess the sequence of potential immunogens to elicit bnAbs, the two organizations will continue to work.
The IAVI’s sponsored JAVI G002 study will enroll 56 HIV negative healthy volunteers at four sites: the George Washington University School of Medicine and Health Sciences (lead investigator David Diemert, M.D.), Hope Clinic of Emory Vaccine Center in Atlanta (lead investigator Srilatha Edupuganti, M.D.), Fred Hutchinson Cancer Research Center (Fred Hutch) in Seattle (lead investigator Julie McElrath, M.D., Ph.D.), and (lead investigator Barbara Taylor, M.D., M.S.).The eOD-GT8 60mer mRNA Vaccine (mRNA-1644) will be given to 48 subjects, with 32 of them getting the boost Core-g28v2 60mer mRNA Vaccine (mRNA-1644v2-Core).
Eight more subjects will get only the boost of immunogen. Participants will be observed for six months following their last vaccine to ensure their safety. The immunological responses of participants to the vaccine candidates will be evaluated in molecular precision to see if the desired responses were obtained.
Lead investigator David Diemert, M.D from the George Washington University School of Medicine and Health Sciences commented:
“We at GWU School of Medicine and Health Sciences are pleased to be part of this endeavor that aims to induce the next step of B-cell maturation toward the goal of generating antibodies that can neutralize a broad range of HIV variants. Further immunogens will be needed to guide the immune system on this path, but this prime-boost combination could be the first key element of an eventual HIV immunization regimen.”
The CAVD Comprehensive Cellular Vaccine Immune Monitoring Consortium/the Dale and Betty Bumpers Vaccine Research Center at the National Institute of Allergy and Infectious Diseases (NIAID)/National Institutes of Health (NIH), the CAVD Comprehensive Antibody Vaccine Immune Monitoring Consortium, Duke University’s Human Vaccine Institute, Fred Hutch, and the Karolinska Institute will perform key analytical assays in support of the trial, to assess whether the targeted immune response is elicited. The CAVD Vaccine Immunology Statistical Center has been constructive, contributing to the study design, analytical methods development, and data evaluation in the study’s conception. Moderna and the Bill and Melinda Gates Foundation signed a global health project framework agreement in January 2016 to accelerate mRNA-based development initiatives for a variety of infectious illnesses. The Bill & Melinda Gates Foundation, the Center for HIV/AIDS Vaccine Immunology and Immunogen Discovery (CHAVI-ID) at NIAID at the NIH, and Moderna supported IAVI and Scripps Research to develop the eOD-GT8 60mer and Core-g28v2 60mer mRNA candidates.
Research at the IAVI NAC that contributed to the development of the vaccine candidates was also made possible by the government of the Netherlands through the Ministry of Foreign Trade & Development Cooperation and through the generous support of the American people through the U.S. President’s Emergency Plan for AIDS Relief (PEPFAR) through the United States Agency for International Development (USAID). The contents are the responsibility of IAVI and Moderna and do not necessarily reflect the views of USAID or the United States government.
About IAVI
IAVI is a nonprofit scientific research organization dedicated to addressing urgent, unmet global health challenges including HIV, tuberculosis, and emerging infectious diseases. Its mission is to translate scientific discoveries into affordable, globally accessible public health solutions. Read more at iavi.org.
About Moderna
Moderna has transformed from a research-stage company advancing programs in the field of messenger RNA (mRNA), to an enterprise with a diverse clinical portfolio of vaccines and therapeutics across seven modalities, a broad intellectual property portfolio in areas including mRNA and lipid nanoparticle formulation, and an integrated manufacturing plant that allows for both clinical and commercial production at scale and at unprecedented speed. Moderna also nurtures alliances with a broad range of domestic and overseas government and commercial collaborators, which has allowed for the pursuit of both groundbreaking science and rapid scaling of manufacturing. Most recently, Moderna’s capabilities have come together to allow the authorized use of one of the earliest and most-effective vaccines against the COVID-19 pandemic.
Moderna’s mRNA platform builds on continuous advances in basic and applied mRNA science, delivery technology and manufacturing, and has allowed the development of therapeutics and vaccines for infectious diseases, immuno-oncology, rare diseases, cardiovascular diseases, and auto-immune diseases. Moderna has been named a top biopharmaceutical employer by Science for the past seven years. To learn more, visit www.modernatx.com.
Recommended Companies
Ad
More Headlines