Daiichi Sankyo’s VANFLYTA® Approved for Acute Myeloid Leukaemia in EU
VANFLYTA® (quizartinib) is now the first FLT3 inhibitor approved in the European Union (EU) specifically for the treatment of patients with newly diagnosed FLT3-ITD positive acute myeloid leukemia (AML).
Japan’s second largest pharma company Daiichi Sankyo announced this week that VANFLYTA® (quizartinib) has been approved in the EU for use in combination with standard cytarabine and anthracycline induction and standard cytarabine consolidation chemotherapy, followed by VANFLYTA single-agent maintenance therapy for adult patients with newly diagnosed AML that is FLT3-ITD positive.
The advancement is grounded on data obtained from the Phase III QuANTUM-First clinical trial evaluating the VANFLYTA® combination regimen. The trial data demonstrated that the treatment offered a 22% reduction in mortality risk versus standard chemotherapy alone.
Ken Keller, Global Head of Oncology Business, and President and CEO, Daiichi Sankyo, Inc. said:
“With the approval of VANFLYTA in the European Union, patients diagnosed with FLT3-ITD positive acute myeloid leukemia may for the first time receive a targeted therapy developed and approved specifically for their disease subtype. VANFLYTA is the second innovative medicine from our oncology pipeline approved in the EU and its successful development reflects our commitment to creating new standards of care for patients with cancer.”
Richard F. Schlenk, MD, Professor and Head of the Trial Center of the National Center of Tumour Diseases, Heidelberg University Hospital and German Cancer Research Center, Germany stated:
“This approval of VANFLYTA represents an important advancement for frontline treatment of patients with FLT3-ITD positive acute myeloid leukemia, an aggressive and historically difficult-to-treat subtype. VANFLYTA is a potent and selective FLT3 inhibitor that significantly improved overall survival when added to standard chemotherapy and it will be a valuable treatment option for newly diagnosed FLT3-ITD positive AML.”
Samantha Nier, Network Director, Acute Leukemia Advocates Network (ALAN) commented:
“This approval of VANFLYTA is very welcome news for eligible patients diagnosed with FLT3-ITD positive AML each year. New medicines and treatment approaches are needed to help patients with this difficult type of leukemia live longer, and we look forward to VANFLYTA becoming available in countries throughout the EU.”
About the QuANTUM-First Trial
The European Commission (EC) authorisation follows the positive opinion of the Committee for Medicinal Products for Human Use and is based on the outcome data of the QuANTUM-First trial, published in The Lancet. QuANTUM-First enrolled 539 patients at 193 study sites in 26 countries across Asia, Europe, North America, Oceania and South America. The primary endpoint of the study was overall survival. Secondary endpoints included event-free survival, post-induction rates of complete remission (CR) and composite complete remission (CRc), and the percentage of patients who achieve CR or CRc with FLT3-ITD measurable residual disease negativity. Safety and pharmacokinetics, along with exploratory efficacy and biomarker endpoints including duration of CR also were evaluated.
In QuANTUM-First, VANFLYTA combined with standard cytarabine and anthracycline induction and standard cytarabine consolidation, and continued as maintenance monotherapy following consolidation, demonstrated a 22% reduction in the risk of death compared to standard chemotherapy alone (HR = 0.78 [95% CI: 0.62-0.98; p=0.032]) in patients with newly diagnosed FLT3-ITD positive AML. Median overall survival was 31.9 months for patients receiving VANFLYTA (n=268; 95% CI: 21.0-NE) compared to 15.1 months for patients in the control arm (n=271; 95% CI: 13.2-26.2) at a median follow-up of 39.2 months.
About VANFLYTA
VANFLYTA is an oral, highly potent type II FLT3 inhibitor that selectively targets FLT3-ITD mutations and has been specifically developed for patients with FLT3-ITD positive AML.
Besides in the EU, VANFLYTA is also approved in the U.S. in combination with standard cytarabine and anthracycline induction and cytarabine consolidation, and as maintenance monotherapy following consolidation chemotherapy, for the treatment of adult patients with newly diagnosed AML that is FLT3-ITD positive as detected by an FDA-approved test. VANFLYTA is not indicated as maintenance monotherapy following allogeneic hematopoietic stem cell transplantation (HSCT); improvement in overall survival with VANFLYTA in this setting has not been demonstrated.
VANFLYTA also is approved in Japan for the treatment of AML that is FLT3-ITD mutation positive, including for use in combination with standard cytarabine and anthracycline induction and standard cytarabine consolidation chemotherapy and as maintenance monotherapy for adult patients with newly diagnosed FLT3-ITD positive AML and as a monotherapy for relapsed/refractory AML that is FLT3-ITD positive as detected by an approved test. VANFLYTA is an investigational medicine in all countries outside of Europe, Japan and the U.S.
For more information, please visit www.daiichisankyo.com
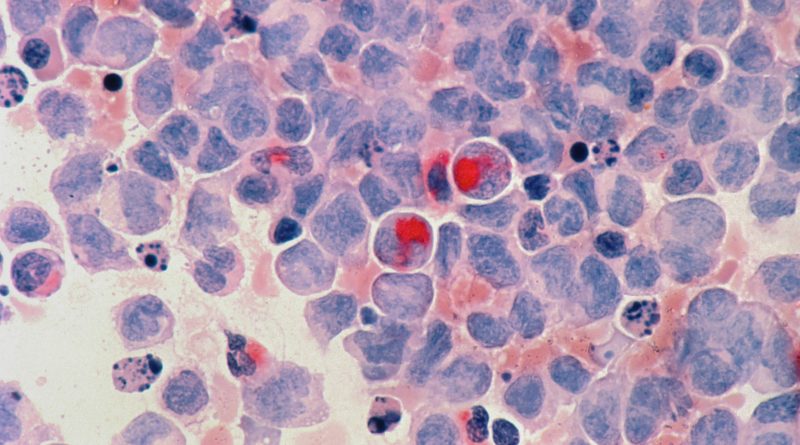
Lead Image Credits: National Cancer Institute, Acute myelocytic leukaemia (AML), Free to use under the Unsplash License
Recommended Companies
More Headlines