Moderna’s ‘Spikevax’ COVID-19 Vaccine Gets Full FDA Approval
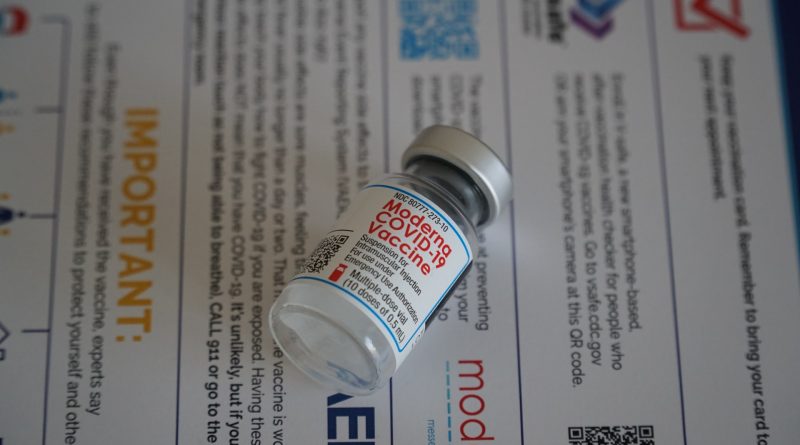
The U.S. Food and Drug Administration (FDA) has approved a second COVID-19 vaccine developed by Moderna, which will be marketed as Spikevax.
The US Food and Drug Administration (FDA) has approved the Biologics License Application (BLA) for SPIKEVAX (COVID-19 Vaccine, mRNA) to prevent COVID-19 in individuals 18 years of age and older, according to Moderna’s announcement released on Monday, a biotechnology company revolutionizing messenger RNA (mRNA) therapeutics and vaccines.
Stéphane Bancel, Chief Executive Officer of Moderna stated:
“Our COVID-19 vaccine has been administered to hundreds of millions of people around the world, protecting people from COVID-19 infection, hospitalization and death. The totality of real-world data and the full BLA for Spikevax in the United States reaffirms the importance of vaccination against this virus. This is a momentous milestone in Moderna’s history as it is our first product to achieve licensure in the U.S. The full licensure of Spikevax in the U.S. now joins that in Canada, Japan, the European Union, the UK, Israel, and other countries, where the adolescent indication is also approved. We are grateful to the U.S. FDA for their thorough review of our application. We are humbled by the role that Spikevax is playing to help end this pandemic.”
More than 70 nations have approved Spikevax
The FDA made its judgment based on the whole body of scientific evidence presented by the Company in its submission package, which included follow-up data from the Phase 3 COVE trial, which showed excellent effectiveness and good safety six months following the second dosage. Moderna also submitted the FDA’s needed production and facility data for license. More than 70 nations, including Canada, Japan, the European Union, the United Kingdom, and Israel, have given their clearance to SPIKEVAX.
Moderna Inc. MRNA shares rose by 4.3% following the announcement
Following the announcement, shares of Moderna Inc. MRNA jumped by 4.3% in early afternoon trading Monday to change hands at $166.20 each. The FDA has also granted full approval to one other COVID-19 shot: BioNTech SE BNTX, +4.37% and Pfizer Inc.’s PFE, +0.72% Comirnaty.
From December 18, 2020, the COVID-19 vaccine from Moderna was accessible in the United States under an Emergency Use Authorization (EUA). When there are no authorized alternatives during a proclaimed public health emergency, the FDA has the right to enable medical items to be used in an emergency to diagnose, treat, or prevent severe or life-threatening illnesses or disorders. Under EUA, a booster dose of the Moderna COVID-19 vaccine at the 50µg dosage level is permitted for emergency use in adults 18 and older in the United States. In the United States, a third dose of the Moderna COVID-19 vaccine at the 100 µg dosage level has been approved for emergency use in immunocompromised adults 18 years of age and older who have been exposed to the virus.
About Moderna’s mRNA platform
Moderna’s mRNA platform builds on continuous advances in basic and applied mRNA science, delivery technology and manufacturing, and has allowed the development of therapeutics and vaccines for infectious diseases, immuno-oncology, rare diseases, cardiovascular diseases and auto-immune diseases. Moderna has been named a top biopharmaceutical employer by Science for the past seven years.
To learn more, visit www.modernatx.com.
Recommended Companies
Ad
More Headlines