Company Categories: CDMO, Cell Line Development, Clinical Diagnostics Equipment, CRO, Drug Development and Supply Chain Equipment
Research & Development of Modern Cell Therapies
DetailsBioinova is a pioneering biotech company specialised in the research and development of modern cell therapies and new diagnostic procedures and methods, production of advanced therapy medicinal products (ATMPs), novel cell storage and transportation solutions, as well as comprehensive clinical research and contract manufacturing services.
Research & Development of Modern Cell Therapies
With its team of highly qualified industry professionals, Bioinova is dedicated to providing patients with innovative, safe, efficient and affordable therapeutic methods in various areas of regenerative medicine and tissue engineering.
Bioinova develops cell therapies that can be used for orthopedic, ophthalmological, neurological, autoimmune indications and as an aid for wound healing. Alongside its cell technologies, the company also develops biomaterials to harness the full potential of stem cells.
New Diagnostic Procedures and Methods
Bioinova’s new company division for pioneering diagnostic procedures and methods has successfully launched its first trademark product – the BiCoVTM set.
The BiCoVTM set is used for sampling and direct RNA isolation and is ready-to-use in PCR testing. Thanks to its novel quality to enable non-invasive sampling and thereby speed-up laboratory diagnostics processes, the BiCoVTM set is quickly gaining recognition and currently seeing increased demand for COVID-19 testing applications.
Bioinova is also expanding its diagnostics pipeline and new products are expected to be launched in the near future.
Contract Manufacturing of Advanced Therapy Medicinal Products (ATMP)
Bioinova operates as a contract manufacturing organisation (CMO) for advanced therapy medicinal products (ATMP). The company develops new state-of-the-art ATMP’s based on stem cells and other cell types obtained and cultured from bone marrow as well as adipose and other tissues.
As a certified manufacturer of ATMP’s Bioinova conducts its production in modern facilities for aseptic processes class A/B under Good Manufacturing Practice (GMP) standards, utilising cutting-edge manufacturing technologies and quality control processes.
Mesenchymal Stromal Cells (MSCs) for Clinical Trials
Bioinova’s core activities involve research, development, production and use of mesenchymal stromal cells also known as mesenchymal stem cells (MSCs) for clinical trials. MSCs play an increasingly important role in the field of regenerative medicine due to their vast therapeutic applications.
Bioinova has successfully developed two patented ATMP’s, the BiCure™ ortho MSCp and BiCure™ ophthalmo MSCs, where the main active component is cultured MSC obtained from bone marrow.
In regenerative medicine, bone marrow-derived mesenchymal stromal cells (BM-MSCs), represent a powerful therapeutic model due to their unique properties, such as ease of isolation from BM avoiding immunological issues, ability to quickly expand in vitro and bio preservation for point-of-care delivery with minimal potency loss. The safety and efficacy of Bioinova’s BM-MSCs is evaluated in preclinical studies and the company’s own clinical trials.
Bioinova combines and tests its products with commercially available, as well as proprietary biomaterials (including nanomaterials), to further enhance their efficacy and to establish new pathways in the field of regenerative medicine and tissue engineering.
Novel Solution for Storage and Transportation of Cells
The freezing and thawing of cells can impact their viability and functional properties. Bioinova has developed a breakthrough patented solution for the storage and transportation of cells-based advanced therapy medicinal products (ATMP’s).
BiCureSol™ is a GMP-compliant solution of excipients for the storage and transportation of cells maintaining cell viability above 92% for a minimum of 72 hours at low temperatures (2-8°C). The novel biological solution allows storage of fresh cells without cryopreservation for up to 120 hours with no loss of cell numbers, viability and phenotype and with the absence of toxic cryopreservants. Bioinova’s tests have shown a clinically relevant quality of mesenchymal stem cells (MSCs) even at up to 10 days of storage.
BiCureSol™ provides sufficient time and ensures reliable and safe transportation of fresh ATMP’s from the manufacturer to the clinical sites even at remote destinations.
Clinical Research Services
Comprised by an expert research team with long-standing experience from leading global research institutions, Bioinova provides comprehensive consultancy and Clinical Research Organization (CRO) services.
Bioinova’s consulting services include GMP operations and assistance with setting up collection and tissue establishments.
As a CRO the company performs clinical trials in accordance with Good Clinical Practice, including monitoring, pharmacovigilance and a comprehensive range of other services.
About Bioinova
Founded in 2008, as a spin-off company of the Institute of Experimental Medicine of the Czech Academy of Sciences, Bioinova has progressed to become a leading privately held biotechnology company in the field of cell-based therapies.
Through the years Bioinova has established a wide international network of partners and scientific and industrial collaborators in the biomedical research, translational medicine and clinical practice fields.
Today, with its dedicated and experienced team, trademark intellectual property, cutting-edge technology and network of reliable partners, Bioinova has the flexibility and knowhow to drive scientific advancement and revolutionize the future of regenerative medicine, tissue regeneration and applied biomedical engineering.
Products
Medium for Storage and Transportation of Cells – BiCureSol®
BiCureSol® is a chemically defined solution for short-term storage and transportation of cells produced under Good Manufacturing Practice (GMP) conditions …
Read More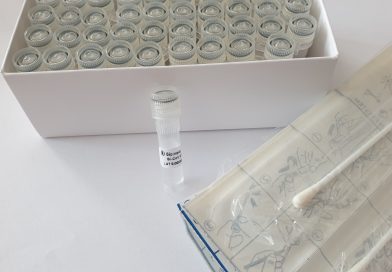
Bi-CoV® Set for COVID-19 Sampling and RNA Isolation
The Set for COVID-19 Sampling and RNA Isolation developed by Bioinova offers high sensitivity and reliability for PCR testing along …
Read MoreDownloadsVideosPress ReleasesContact


