FDA Greenlights Iovance’s AMTAGVI™ as First T-cell Therapy for Solid Tumor Cancer
Iovance Biotherapeutics, Inc., a leading biotechnology firm specialising in pioneering, developing, and delivering advanced polyclonal tumor infiltrating lymphocyte (TIL) cell therapies for cancer patients, announced a significant milestone. The U.S. Food and Drug Administration (FDA) has approved AMTAGVI™ (lifileucel) suspension for intravenous infusion as the first T cell therapy for a solid tumor cancer and first treatment option for advanced melanoma after anti-PD-1 and targeted therapy.

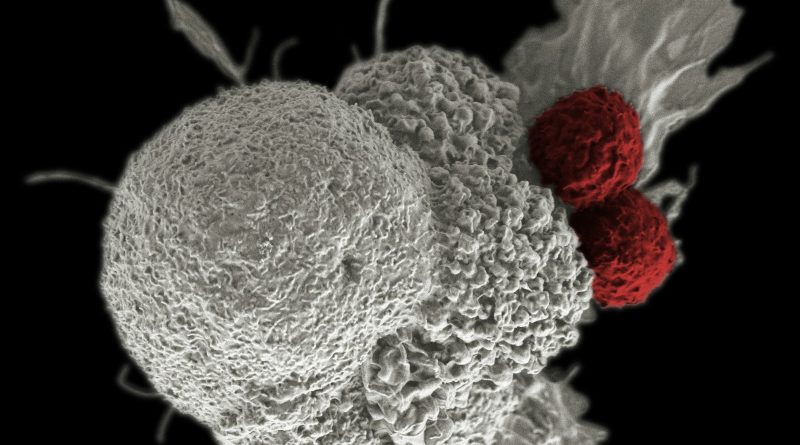
This groundbreaking therapy is a tumor-derived autologous T cell immunotherapy designed for adult patients facing unresectable or metastatic melanoma, particularly those who have previously undergone treatment with a PD-1 blocking antibody. Additionally, it is indicated for patients with a BRAF V600 mutation positive status, with or without a MEK inhibitor. This regulatory approval, granted under accelerated approval, is based on compelling data demonstrating overall response rate (ORR) and duration of response.
AMTAGVI marks a significant advancement in personalized cancer treatment, being the first and only individualized T cell therapy to gain FDA approval for a solid tumor cancer. The therapy operates through a unique mechanism that harnesses patient-specific TIL cells. These cells, naturally occurring in the immune system, are mobilized to identify, target, and eliminate cancer cells bearing distinctive tumor markers. However, in the presence of advanced cancer, the body’s natural TIL cells often lose their effectiveness in combating the disease.
Manufactured through a proprietary process, AMTAGVI involves the collection and expansion of a patient’s own unique T cells obtained from their tumor tissue. Following this process, billions of these reinvigorated T cells are reintroduced into the patient’s body, thereby enhancing the immune response against their cancer. The therapy will be administered by Authorized Treatment Centers (ATCs) as part of a comprehensive treatment regimen, which includes lymphodepletion and a short course of high-dose PROLEUKIN® (aldesleukin).
Frederick Vogt, Ph.D., J.D., Interim Chief Executive Officer and President of Iovance states:
“The accelerated approval of AMTAGVI™ is the first step in realizing Iovance’s ambition to usher in the next generation of cell therapy by bringing this breakthrough to patients with advanced solid tumors. Given the significant unmet needs in the advanced melanoma community, we are proud to offer a personalized, one-time therapeutic option for these patients. We are continuing our development efforts to address additional unmet medical needs in patients with solid tumor cancers, making our novel cell therapies available to more patients with melanoma and other types of cancers.”
Samantha R. Guild, J.D., President, AIM at Melanoma Foundation comments:
“The approval of AMTAGVI™ offers hope to those with advanced melanoma who have progressed following initial standard of care therapies, as the current treatment options are not effective for many patients. This one-time cell therapy represents a promising innovation for the melanoma community, and we are excited by its potential to transform care for patients who are in dire need of additional therapeutic options.”
Furthermore, Iovance is actively engaged in advancing its clinical research efforts with TILVANCE-301, a Phase 3 trial aimed at validating the clinical benefits of this groundbreaking therapy. This latest FDA approval underscores Iovance’s commitment to pioneering innovative treatments that hold the promise of transforming the landscape of cancer care.
For more information, please visit www.iovance.com.
Original Source: Press Release: Feb. 16, 2024 (GLOBE NEWSWIRE) Iovance’s AMTAGVI™ (lifileucel) Receives U.S. FDA Accelerated Approval for Advanced Melanoma
Recommended Companies
More Headlines