Roche’s Actemra (tocilizumab) shows “excellent effects” against COVID-19
The widely used arthritis medicine Actemra (tocilizumab) has demonstrated “excellent effects” in the treatment of the COVID-19 disease.
Roche’s promising arthritis drug Tocilizumab is being used to treat coronavirus (COVID-19) patients in China and has helped to cure 90.5 per cent of critically ill patients, scientists claim in a study supported by the China National Centre of Biotechnology.
Roche’s Actemra ® (tocilizumab) was first licenced by the US Food and Drug Administration (FDA) in 2010, as an alternative treatment for adult patients with mild to extreme active rheumatoid arthritis (RA) and Giant cell arteritis (GCA).
The Actemra/RoActemra (tocilizumab) medicine is now approved in 116 countries for the treatment of rheumatoid arthritis (RA), as well as for the treatment of paediatric juvenile idiopathic arthritis (pJIA), systemic juvenile idiopathic arthritis (sJIA), giant cell arteritis (GCA) and CAR-T cell-induced cytokine release syndrome (CRS). Actemra/RoActemra is available in both subcutaneous (SC) and intravenous (IV) formulations.
Modern approaches to treat the excessive immune response for a range of rheumatic, oncological and other inflammatory autoimmune conditions include drugs that target interleukin-1(IL-1), interferon-gamma, IL-18 and IL-6. These drugs target one or a few inflammatory molecules, including cytokines, without triggering general immunosuppression, unlike the other relatively non-selective immunosuppressants such as corticosteroids.
Treating cytokine storms brought about by other illnesses, like other viral infections and autoimmune diseases, death rates among patients suffering an extensive immune response have been reduced to as low as 27 percent.
Actemra (tocilizumab) is capable of inhibiting elevated rates of protein Interleukin 6 (IL-6), triggered by inflammatory diseases including COVID-19 cases with complications.
China’s Tocilizumab clinical trial shows positive results
Previously, IL-6 blockade has been confirmed to be in use in China with positive outcomes in certain individuals undergoing this as part of their COVID-19 complication therapy.
Actemra was used in an initial clinical trial in 20 extreme COVID-19 cases in China. According to China’s National Health Commission, nineteen of the patients were released from hospital within two weeks and one got better. Moreover, the CT scans also show lung damage greatly decreased on the fourth and fifth day of treatment.
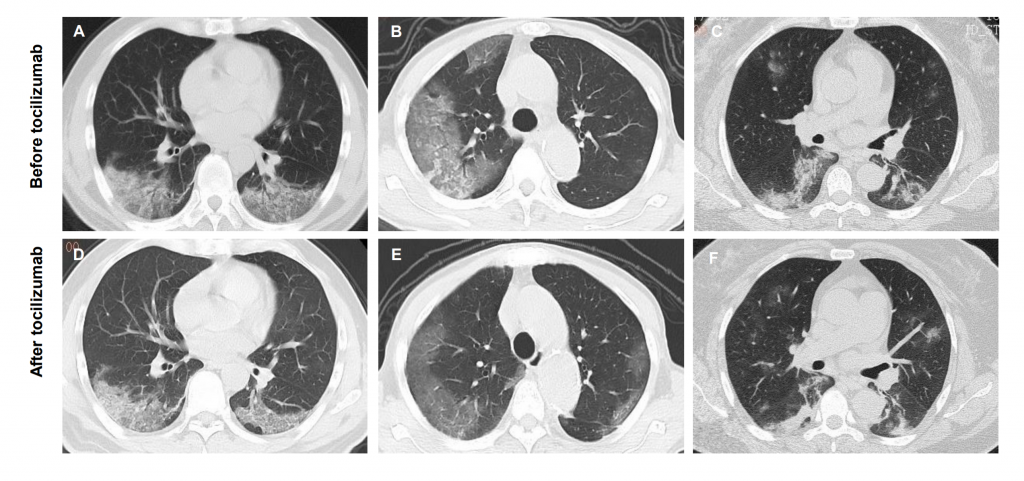
The authors of the study concluded:
‘Tocilizumab is an effective treatment in severe patients of COVID-19, which provided a new therapeutic strategy for this fatal infectious disease.’
Actemra (tocilizumab) is officially approved for COVID-19 treatment in China
While novel and repurposed antiviral therapies are being investigated for the treatment of COVID-19, the Swiss healthcare group Roche has obtained China’s approval of its anti-inflammatory medication Actemra (tocilizumab) for treating patients with serious coronavirus complications (Covid-19), Reuters reported.
On the 3rd of March, China’s National Health Commission released a clinical recommendation that allows the use of Actemra (tocilizumab) to treat patients with the novel coronavirus who have elevated rates of IL-6 and who have severe lung damage.
Actemra (tocilizumab) shows success in Italy
Actemra has also made head waves in Italy. The Italian oncologist Paolo Ascierto of Pascale Hospital in Naples said earlier this month that, Actemra (tocilizumab) has demonstrated “excellent effects” in two patients with coronavirus and a national guideline will be drawn up for its systematic use against the virus, Italian news outlet ANSA reported.
Paolo Ascierto further commented for ANSA, that Roche’s drug Actemra (tocilizumab), “has shown it is effective against pneumonia caused by COVID-19” and one of the two patients will be taken off life support because of the improvement in his condition. He also called for a “national protocol to immediately extend the use of tocilizumab in an emergency”.
Italian cardiologist Giuseppe Galati tweeted about Tocilizumab :
Roche initiates Actemra (tocilizumab) Phase 3 clinical trial
Karsten Kleine, a spokesperson for Roche, told NBC News that the pharmaceutical company ‘is in active discussions with the FDA, as well as government bodies and institutions around the world, to initiate clinical trials that evaluate the safety and efficacy of Actemra (tocilizumab) for the treatment of severely ill COVID-19 patients.’
On the 19th March Roche announced that the company is working with the FDA to initiate a randomised, double-blind, placebo-controlled Phase 3 clinical trial in collaboration with the Biomedical Advanced Research and Development Authority (BARDA), to determine the safety and efficacy of Actemra®/RoActemra® (tocilizumab) plus standard of care in hospitalised adult patients with severe COVID-19 pneumonia compared to placebo plus standard of care.
According to Genentech, a member of the Roche Group and the manufacturer of the interleukin-6 receptor antagonist, an additional 10,000 virals of Actemra will be provided to the U.S. Strategic National Stockpile for potential future use at the direction of the U.S. Department of Health and Human Services (HHS).
Alexander Hardy, CEO of Genentech, said in the companies press release form 23rd March that:
‘We thank the FDA for rapidly expediting the approval of this clinical trial to evaluate Actemra in critically ill patients suffering from pneumonia following coronavirus infection and we’re moving forward to enroll as quickly as possible.’
Approximately 330 patients in the United States and other countries will be monitored for 60 days, with recruitment expected to start in early April. The primary and secondary endpoints of the trial will include clinical status, mortality, mechanical ventilation and intensive care unit (ICU) variables.
How Tocilizumab works
The critically significant studies coming from China indicate that it may be the patient’s own immune system overresponse, rather than the virus itself, which triggers fatal respiratory complications for many patients who die of Covid-19. This syndrome is well known as a “storm of cytokines” and is typically fatal without treatment.
It represents an excessive immune response leading to an enormous cytokine release which causes extensive damage and dysfunction of the healthy lung tissue. The Cytokine outbreak is considered as the main cause resulting in acute respiratory distress and multiorgan failure.
The elevated values of Interleukin 6 (IL-6), have recently been identified in Covid-19 patients hospitalised in China. It is essential that there is an available, inexpensive and fast blood test, Interleukin 6 (IL-6), which is a good initial screening marker for the risk of developing a cytokine storm syndrome. This could help to identify and diagnose deteriorating patients and substantially reduce COVID-19 fatal complications.
Providing patients with an anti-IL-6 receptor therapy inhibits the extensive immune response that escalates to catastrophic multi-organ dysfunction syndrome (MODS) and RDS respiratory distress syndrome.
Roche’s Actemra/RoActemra (tocilizumab) is a first-in-class anti-IL-6 receptor (aIL-6R) therapy. IL-6 is believed to play a key role in activating the inflammatory pathway that contributes to the signs and symptoms of RA and other inflammatory autoimmune conditions. Actemra/RoActemra works by binding to IL-6 receptors and blocking the pro-inflammatory effect of IL-6 cytokines.