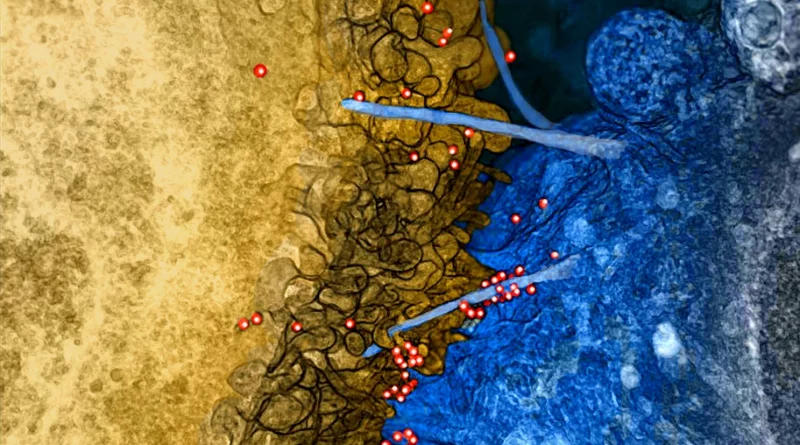
FDA Approves J&J and Bristol Myers Cell Therapies for Refractory Multiple Myeloma
The U.S. Food and Drug Administration has approved the use of cell therapies developed by Johnson & Johnson (JNJ.N) and Bristol Myers Squibb (BMY.N) for treating patients in the early stages of a type of with refractory multiple myeloma, a type of blood cancer hat doesn’t get better with treatment or that stops responding to treatment. According to separate statements from both companies, these therapies demonstrated better disease progression outcomes than the standard of care treatments in late-stage studies, extending the time that patients lived without disease progression.
The FDA’s approval for using the therapies — J&J’s CARVYKTI®and Bristol Myers’ Abecma® — in less severely affected patients with multiple myeloma follows an endorsement by an expert panel last month.
On Friday, Johnson & Johnson announced that the U.S. FDA has approved CARVYKTI® (ciltacabtagene autoleucel; cilta-cel) for the treatment of adult patients with relapsed or refractory multiple myeloma who have received at least one prior line of therapy, including a proteasome inhibitor and an immunomodulatory agent, and are refractory to lenalidomide. With this approval, J&J’s CARVYKTI® becomes the first and only B-cell maturation antigen (BCMA)-targeted therapy approved for the treatment of patients with multiple myeloma as early as the first relapse. CARVYKTI® is a cell therapy that works by harnessing a patient’s immune system, or T cells, to fight the disease. The expanded indication for this one-time infusion is set to provide more patients with a potential period away from their multiple myeloma treatment as early as first relapse.
Bristol Myers Squibb and 2seventy bio, Inc. announced on April 4, 2024, the U.S. FDA approved Abecma® (idecabtagene vicleucel; ide-cel) for the treatment of adult patients with relapsed or refractory multiple myeloma after two or more prior lines of therapy including an immunomodulatory agent (IMiD), a proteasome inhibitor (PI), and an anti-CD38 monoclonal antibody. This approval expands Abecma’s indication, making it available in earlier lines to patients who have relapsed or become refractory after exposure to these three main classes of treatment (triple-class exposed), after two prior lines of therapy. The expanded approval now makes this CAR T cell therapy available to a broader group of patients with relapsed or refractory multiple myeloma at an earlier stage in their personalised treatment process. Administered as a one-time infusion, it provides significant treatment-free intervals when patients respond to the therapy. Furthermore, Abecma has received approval in the U.S., Japan, Switzerland, and the EU for use in patients with triple-class exposed relapsed and/or refractory multiple myeloma. This underscores Bristol Myers Squibb’s commitment to making Abecma accessible globally, with consistently high manufacturing success rates and continuous capacity expansion.
Recommended Companies
More Headlines