The Role of Microglia and T Cells in Age-Associated Decline
A recent study by Janos Groh and colleagues in Mikael Simons’ group at the Institute of Neuronal Cell Biology, Technical University of Munich, examines how immune mechanisms contribute to white matter degeneration during ageing. Featured in the monthly scientific journal Nature Neuroscience, the research highlights the role of activated microglia in promoting neuroinflammation and uncovers a damaging cascade mediated by cytotoxic CD8⁺ T cells. At the core of this process is the CXCL10–CXCR3 signaling pathway. These results delineate a molecular pathway that may drive persistent immune activation in age-related white matter degeneration.

PUBLICATION
Nature Neuroscience (Jun 28, 2025) ”Microglia activation orchestrates CXCL10-mediated CD8+ T cell recruitment to promote aging-related white matter degeneration”
Groh J, Feng R, Yuan X, Liu L, Klein D, Hutahaean G, Butz E, Wang Z, Steinbrecher L, Neher J, Martini R, Simons M.
Microglial Dysfunction and Its Impact on Ageing White Matter
White matter in the ageing brain is particularly susceptible to injury. White matter consists of regions in the brain and spinal cord largely made up of axons, the nerve fibers coated in myelin, which gives the tissue its distinctive white colouration. Myelin sheaths, generated by oligodendrocytes, gradually accumulate reactive oxygen species with advancing age, resulting in structural defects such as decompaction, fragmentation, and eventual axonal damage. These alterations are linked to reduced integrity of axonal tracts and, in turn, cognitive decline in older individuals.
As in many degenerative disorders, certain aspects of this process can be traced to an inappropriate immune response, whether an insufficient, excessive, or misdirected reaction. In this instance, the critical contributors are microglial cells and CD8⁺ T cells.
Microglia, the resident macrophages of the CNS, normally play an essential role in sustaining white matter homeostasis by clearing damaged myelin. With ageing, however, this system becomes compromised, as the phagocytic ability of microglia cannot match the rate of ongoing myelin degeneration. This imbalance gives rise to chronic neuroinflammation, in which persistent myelin abnormalities drive a shift in microglial state. They acquire transcriptional profiles resembling disease-associated, or “reactive,” microglia, marked by heightened pro-inflammatory signaling, increased lysosomal content, lipofuscin-filled vesicles, and distinctive pathological hallmarks including cytoplasmic spheroids and beading.
Crucially, these reactive microglia are frequently observed together with CD8⁺ T cells in aged or pathological CNS tissue. Instead of alleviating damage, CD8⁺ T cells appear to exacerbate it by promoting axonal degeneration and oligodendrocyte injury. This consistent co-occurrence raises important questions regarding how reactive microglia and CD8⁺ T cells interact within white matter regions.
Modeling Age-Related Dysregulation of Microglial Activation in the Optic Nerve
To investigate this interaction, the researchers chose the optic nerve as their experimental model system. Its anatomical properties, along with the ability to behaviorally assess optic nerve integrity through visual acuity testing with Striatech’s OptoDrum device, make it a suitable proxy for ageing white matter.
The study employed two distinct models of age-related microglial disturbance, each leading to an increased proportion of reactive microglia. In the first, pharmacological manipulation with PLX5622 was used to enhance microglial activation. PLX5622 targets microglial survival pathways, depletes CSF1R-dependent microglia, and preferentially reduces homeostatic microglial populations. The second, a genetic model, involved CX3CR1-deficient mice. CX3CR1 encodes a chemokine receptor associated with neuroprotective, anti-inflammatory microglial states; its deletion predisposes microglia to pro-inflammatory activation.
Both approaches resulted in a higher prevalence of CD11c-expressing microglia in aged optic nerves. CD11c serves as a marker of transition toward activated microglial phenotypes. Electron microscopy revealed overlapping pathological features, including myelin fragmentation, axonal spheroids, and cellular debris accumulation. In PLX5622-treated mice, microglia displayed intracellular myelin remnants and enlarged lysosomal inclusions, indicative of ongoing but inefficient debris clearance. By contrast, CX3CR1-deficient microglia were found in contact with abnormal myelin but did not phagocytose it.
These structural abnormalities in the optic nerve coincided with thinning of the inner retina, loss of retinal ganglion cells (RGCs), and marked reductions in visual acuity in both models, as measured using Striatech’s OptoDrum system.
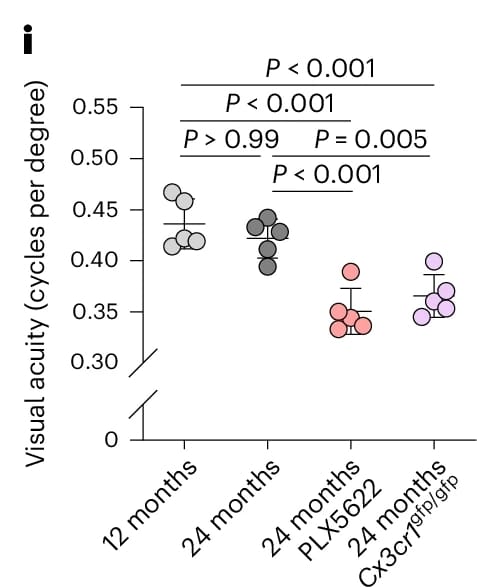
Measurements with Striatech’s OptoDrum showcase that visual acuity is significantly reduced in aged mice in both experimental models.
Reproduced from Figure 2 of the original publication under the Creative Commons Attribution (CC BY 4.0) license.
Single-cell RNA Sequencing Identifies Diverse Microglial Heterogeneity
To investigate the molecular mechanisms underlying these findings, the researchers conducted single-cell RNA sequencing (RNA-seq) of microglia. Analysis of aged white matter revealed three distinct microglial populations. The first cluster was characterised by elevated expression of canonical inflammatory genes. The second cluster was enriched for transcripts linked to phagocytosis, oxidative phosphorylation, and ribosomal function. A third cluster, which was particularly prominent in aged PLX5622-treated mice, displayed a mixed profile, combining high levels of inflammatory markers with genes involved in antigen presentation and T cell interactions.
In addition to microglial changes, other glial populations also showed stress-associated adaptations. Oligodendrocytes, the myelin-forming cells, upregulated genes related to DNA damage responses and protein stress. Astrocytes developed reactive transcriptional profiles, highlighted by increased GFAP expression. Collectively, these findings demonstrate that microglial dysfunction propagates wider disturbances across the glial network.
CD8⁺ T Cells Mediate White Matter Damage During Ageing
Both experimental approaches that altered microglial function in aged white matter led to pronounced accumulation of CD8⁺ T cells in proximity to CD11c-positive microglia. These T cells exhibited characteristics of tissue-resident memory (TRM) cells, including granzyme B expression, indicative of cytotoxic potential. Moreover, CD8⁺ T cells were spatially associated with axonal spheroids and reactive oligodendrocytes, suggesting an active contribution to tissue injury.
To test this hypothesis, the authors generated aged CD8-deficient mice lacking functional CD8⁺ T cells and subjected them to PLX5622 treatment. This allowed separation of microglial depletion effects from T cell involvement. While myelin abnormalities and microglial CD11c expression persisted, key indicators of neurodegeneration, including axonal spheroids, retinal ganglion cell loss, and retinal thinning, were markedly reduced. Visual acuity, measured via Striatech’s OptoDrum, was also significantly improved compared to CD8-sufficient controls. These results confirm a causal role for CD8⁺ T cells in exacerbating glia-induced white matter degeneration.
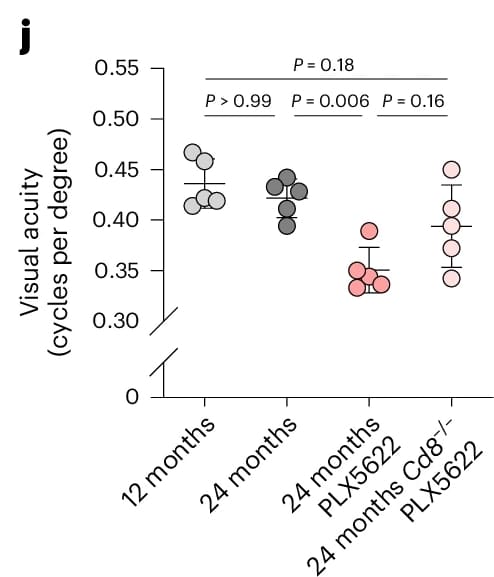
The visual acuity of aged CD8-deficient mice was less affected by PLX5622 treatment.
Reproduced from Figure 5 of the original publication under the Creative Commons Attribution (CC BY 4.0) license.
The CXCL10–CXCR3 Signaling Pathway Connects Glial Activation to T Cell Recruitment
To uncover the molecular signals responsible for CD8⁺ T cell recruitment, spatial transcriptomics using MERFISH (Multiplexed Error-Robust Fluorescence In Situ Hybridization) was employed. This technique allows simultaneous mapping of over 500 mRNAs at single-cell resolution while preserving spatial information.
MERFISH analysis demonstrated an overall reduction in microglial numbers following PLX5622 treatment, consistent with partial depletion, alongside increased T cell infiltration. The chemokine CXCL10 was expressed across multiple glial cell types, including astrocytes and microglia. CD8⁺ T cells were spatially co-located with CXCL10-expressing cells, supporting its role as a chemotactic signal. Gene expression profiling of the T cells confirmed robust expression of CXCR3, the cognate receptor for CXCL10.
From these data, the authors proposed a three-component model: (1) CD11c-positive microglia initiate inflammatory responses, (2) reactive astrocytes and microglia amplify this signal via upregulation of CXCL10, and (3) CXCR3-positive CD8⁺ T cells are recruited to the site, where they mediate cytotoxic damage.
Genetic Validation of the Ligand–Receptor Pathway
Sophisticated transgenic mouse models were employed to test this model. Trem2-deficient mice, whose microglia remain in homeostatic, non-reactive states, were used to determine whether microglial activation is required for CXCL10 upregulation and CD8⁺ T cell infiltration. These mice exhibited reduced CD11c expression, lower CXCL10 levels, and fewer CD8⁺ T cells, in spite of the presence of myelin disruption.
Next, aged CXCL10-deficient mice were examined. While microglial activation and myelin damage were comparable to controls, these animals displayed markedly reduced CD8⁺ T cell infiltration, fewer axonal spheroids, preservation of retinal ganglion cells, and maintained visual acuity. This confirmed that CXCL10 is essential for T cell recruitment and persistence in ageing white matter.
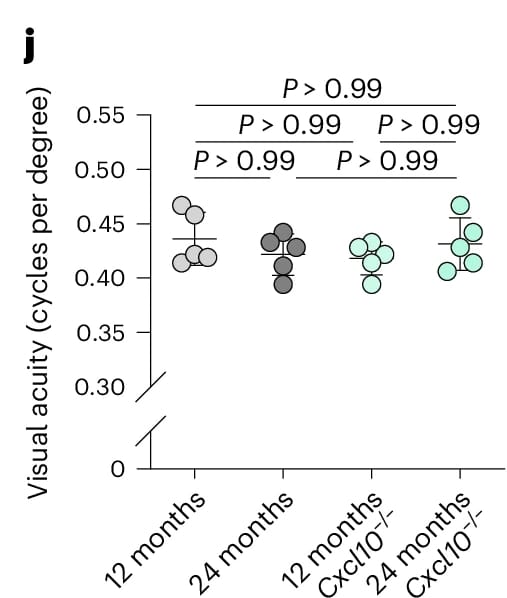
CXCL10-deficient mice do not experience age-dependent visual decline.
Reproduced from Figure 7 of the original publication under the Creative Commons Attribution (CC BY 4.0) license.
To assess the role of CXCR3 on T cells, Rag1−/− mice lacking adaptive immunity were reconstituted with either wild-type or CXCR3-deficient bone marrow. Only mice receiving wild-type (CXCR3-competent) cells developed substantial T cell infiltration, axonal damage, and persistent CD8⁺ T cell presence in the CNS.
Impact of Glia–T Cell Interactions on Aging White Matter
This study recognises the CXCL10–CXCR3 signaling pathway as a mechanistic link between microglial dysfunction and CD8⁺ T cell-driven neurodegeneration in the ageing CNS. The research findings indicate that CD8⁺ T cells are misdirected by glial signals, contributing to a cycle of persistent inflammation and axonal injury.
Conversely to conventional immune responses directed against pathogens, this glia–T cell interaction appears maladaptive in the ageing brain. Activated glia may present myelin-derived, age-associated antigens, driving ongoing T cell engagement and tissue damage.
Therapeutically, targeting this pathway, by modulating microglial activation, blocking CXCL10 or CXCR3, or limiting T cell persistence, could provide a strategy to preserve white matter integrity and maintain cognitive function during aging.
Original Source: Emilia Kawecka, Technical University of Munich, Student Assistant at Striatech
Original Paper: Groh, J., Feng, R., Yuan, X. et al. Microglia activation orchestrates CXCL10 mediated CD8+ T cell recruitment to promote ageing-related white matter degeneration. Nat Neurosci 28, 1160–1173 (2025). https://www.nature.com/articles/s41593-025-01955-w
Related Product