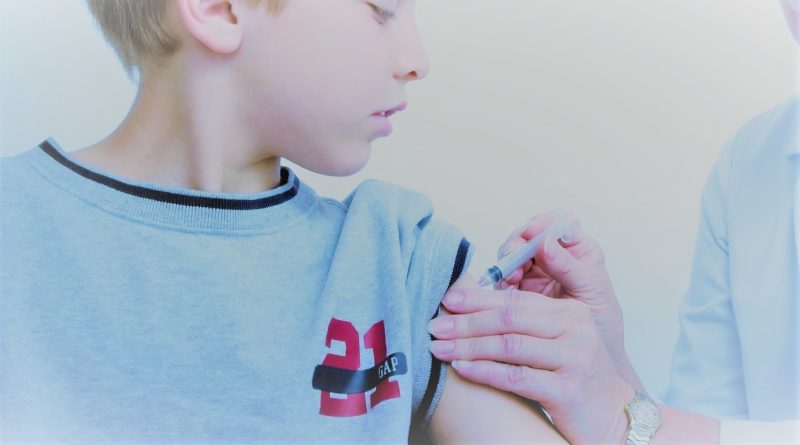
Pfizer-BioNTech Vaccine Approved in the UK for 12 to 15-year-olds
The Medicines and Healthcare products Regulatory Agency (MHRA) has approved the use of the Pfizer-BioNTech vaccine in children aged 12-15 in the United Kingdom.
MHRA concludes “benefits of the vaccine outweigh the risks”
This comes after a thorough examination of the vaccine’s safety, quality, and effectiveness in this age range by the MHRA and the Government’s independent advisory body, the Commission on Human Medicines (CHM).
Dr June Raine, the MHRA Chief Executive stated:
“We have carefully reviewed clinical trial data in children aged 12 to 15 years and have concluded that the Pfizer/BioNTech Covid-19 vaccine is safe and effective in this age group and that the benefits of this vaccine outweigh any risk.”
The Pfizer-BioNTech COVID-19 vaccine is already approved for use in adults and adolescents aged 16 years and above. Over 2,000 children aged 12 to 15 were studied as part of the randomised, placebo-controlled clinical trials. The vaccinated group had no cases of the virus seven days after the second dosage, compared to 16 instances in the placebo group. Furthermore, data on neutralizing antibodies revealed that the vaccine was effective at the same level as in adults aged 16 to 25. There were no additional adverse effects discovered, and the safety data in youngsters was comparable to that of young adults.
Professor Sir Munir Pirmohamed, Chair of the Commission on Human Medicines said:
“We have concluded that based on the data we have seen on the quality, effectiveness and safety of the vaccine, its benefits do outweigh any risk. The MHRA will continue to scrutinise all of the suspected side effects data received through the rigorous surveillance programme in place through the Yellow Card scheme and other safety surveillance measures for all of the COVID-19 vaccines used in the UK.”
JCVI to consider whether to vaccinate children
The Joint Committee on Vaccination and Immunisation (JCVI), a government advisory body, will now consider the evidence before making a decision on whether or not to give the vaccination to children.
Dr June Raine confirmed:
“It will now be for the Joint Committee on Vaccination and Immunisation (JCVI) to advise on whether this age group will be vaccinated as part of the deployment programme.”
The UK’s Health Secretary Matt Hancock tweeted:
Mr Hancock explained that any decision would be “clinically based” and confirmed that the UK had sufficient supplies to vaccinate children if recommended to by the JCVI.
The Pfizer-BioNTech vaccine for 12 to 15-year-olds was approved by the European Union a week ago, and vaccinations for youngsters in this age group have already started earlier this month in the United States and Canada.
Recommended Companies
Ad
More Headlines