Pfizer and BioNTech Start Phase 1/2 Study of First mRNA-based Shingles Vaccine in the US
Pfizer and BioNTech plan to use mRNA technology to meet the demand for highly effective, well-tolerated, and effectively manufactured shingles vaccines as the world’s population ages. Up to 900 healthy American volunteers aged 50 to 69 are expected to participate in the phase 1/2 study.
Pfizer Inc. and BioNTech SE announced on Friday, the beginning of a Phase 1/2 study investigating the safety, tolerability, and immunogenicity of the companies’ mRNA vaccine candidates against shingles, also known as herpes zoster (or “HZ”). Shingles is a disabling condition brought on by the varicella-zoster virus (VZV). Each year, shingles affects millions of individuals worldwide.
The companies’ Phase 1/2 multicenter, randomized, controlled, dose-selection trial (NCT05703607) will evaluate the immunogenicity, safety, and tolerance of the candidate mRNA shingles vaccines.
Up to 900 volunteers to participate in mRNA shingles vaccine candidate Phase 1/2 study
Up to 900 healthy volunteers between the ages of 50 and 69 are enrolled in the study, which will be conducted in the United States. Phase 1 will help in selecting the best mRNA vaccine candidate, dosage level, dosage schedule, and formulation for advancement to Phase 2. Participants in the study will be monitored to determine the length of potential protection.
Despite the fact that there are existing registered shingles vaccines, Pfizer and BioNTech intend to leverage mRNA technology to perhaps develop a vaccine with high efficacy, good tolerability, and low cost of production globally. Both Pfizer’s proprietary antigen technology and BioNTech’s patented mRNA platform technology will be used by the companies that created the COVID-19 vaccine.
The many forms of the glycoprotein E (“gE”) on the surface of the varicella-zoster virus are encoded by the mRNA shingles vaccine candidates. The gE protein is essential for viral replication and cell-to-cell transmission following the virus’ reactivation in nerve cells. In January 2022, Pfizer and BioNTech announced a partnership to develop a shingles vaccine. Additionally, the businesses are working on a COVID-19 and influenza combo vaccination program.
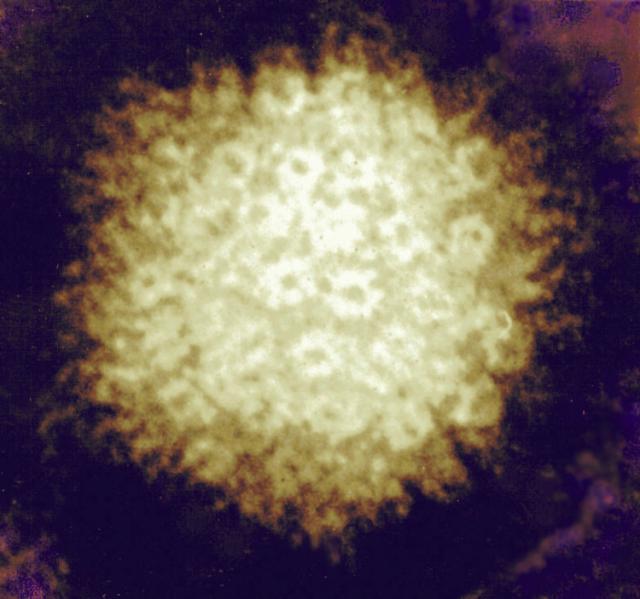
Firey-looking electron micrograph of herpes simplex virus. Credit: NIAID (http://www.flickr.com/photos/niaid/5614251360/)
About Shingles (Herpes Zoster)
Globally, 95% of people over 50 have been exposed to the VZV, putting them at risk for acquiring shingles. Additionally, as people age, shingles become more common and more severe, with a dramatic increase occurring after age 50.A shingles vaccine is an essential worldwide health concern due to an aging global population.
Herpes zoster, often known as shingles or “HZ,” is an infectious condition brought on by the varicella zoster virus. Chickenpox is typically caused by a primary VZV during childhood. The virus remains latent in human nerve cells after chickenpox, and can subsequently get reactivated by immune suppression or stress.