Lilly starts Phase 3 Trial of monoclonal antibody for COVID-19 prevention
Eli Lilly and Company announced the start of BLAZE-2, a Phase 3 study of the monoclonal antibody LY-CoV555 vaccine candidate component for prevention of SARS-CoV-2 infection and COVID-19 in residents and staff of U.S. nursing homes and assisted living facilities. The first-of-its-kind clinical trial, held in partnership with the National Institute of Allergy and Infectious Diseases (NIAID), will enrol up to 2,400 residents and staff.
First-of-a-kind monoclonal antibody trial against SARS-CoV-2
The lead antibody in Lilly’s alliance with AbCellera, LY-CoV555, is a neutralising monoclonal antibody against SARS-CoV-2, the virus that triggers COVID-19. The high infection rates of SARS-CoV-2 amongst occupants in long-term care facilities along with increased mortality levels for the elderly presents, present an immediate need for treatment to prevent COVID-19 in this vulnerable demographic population.
Daniel Skovronsky, M.D., Ph.D., Lilly’s Chief Scientific Officer and President of Lilly Research Laboratories stated in the companies press release published on 3rd April:
“COVID-19 has had a devastating impact on nursing home residents. We’re working as fast as we can to create medicines that might stop the spread of the virus to these vulnerable individuals. While it’s not easy to conduct clinical trials in this setting, we’re taking on the challenge in an effort to help those who need us the most. We are grateful to the NIAID team for their exceptional partnership on this innovative trial and we are deeply appreciative of the care facilities, their staff and the many residents who will be participating in this important study.”
The unprecedented Lilly-sponsored BLAZE-2 study is being performed in partnership with NIAID, part of the National Institutes of Health (NIH), the COVID-19 Prevention Network (CoVPN) and several long-term care facilities across the country.
Up to 2400 participants in the high-risk category
The late-stage clinical trial is set to recruit up to 2400 occupants and personnel who live or operate in facilities which have recently had a confirmed case of COVID-19 and are already at an elevated risk of exposure.
The trial is aiming to assess the effectiveness and safety of a single dose LY-CoV555 and weather it is going to be efficient enough to reduce the risk and to prevent SARS-CoV-2 infection over 4 weeks, as well as the complications of the COVID-19 disease over 8 weeks.
Myron Cohen, M.D., director of UNC’s Institute for Global Health and Infectious Diseases and a CoVPN leader stated:
“The mission of the COVID-19 Prevention Network is to conduct Phase 3 vaccine and monoclonal antibody efficacy studies for the prevention of COVID-19. We’re excited to partner with Lilly to determine whether LY-CoV555 can prevent or mitigate progression of COVID-19 infection in this vulnerable population that has been greatly impacted by this virus.”
Mobile labs and on-site analysis
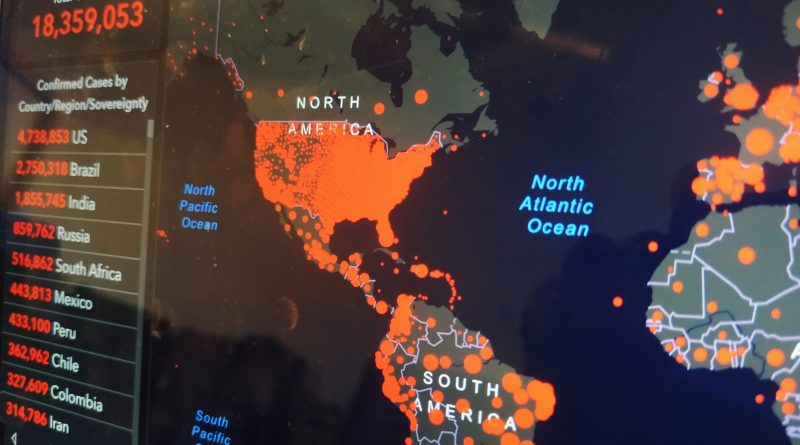
More than 4.5 million U.S residents have so far been confirmed to have COVID-19. In order to resolve the daunting challenges of conducting clinical research in a long-term care center amidst a pandemic, Lilly has developed specialised mobile testing units to help the on-site analysis. These units include a custom retrofitted recreation vehicle (RV) to support mobile labs and clinical trial material preparation, along with a trailer truck to deliver all the clinical trial supplies needed to create an on-site infusion clinic. In order to further minimise the strain on these facilities, which do not normally carry out clinical studies, extra staff will be allocated to the facilities to support the study. Eli Lilly will utilise its mobile research unit fleet responding to the virus outbreaks in long-term care facilities across the United States.
Dr. Alexander Stemer, a board certified infectious disease expert and co-chair of Symphony Care Network’s COVID-19 task force said:
“We commend Lilly and NIH in recognising the critical need for developing medicines to combat the spread of the virus among some of the most vulnerable populations. As the scientific community searches for safe and effective therapies for COVID-19, we are proud to participate in this leading-edge study given its potential to save lives.”
About the LY-CoV555 neutralising IgG1 monoclonal antibody
LY-CoV555 is an active, neutralising IgG1 monoclonal antibody (mAb) directly targeting the SARS-CoV-2 spike protein. It is engineered to inhibit viral attachment and entrance into human cells, thus neutralising the virus and preventing and treating COVID-19. LY-CoV555 emerged from the cooperation between Eli Lilly and AbCellera to develop an antibody therapy for prophylactic and treatment of COVID-19.
Lilly researchers created the antibody very quickly, in less than three months after it was discovered by AbCellera and tested by scientists at the NIAID Vaccine Research Centre. It was detected in a blood sample taken from one of the first U.S. patients to have recovered from COVID-19. Eli Lilly successfully accomplished dosing of LY-CoV555 in a Phase 1 trial of hospitalised patients with COVID-19 (NCT04411628) and long-term follow-up is underway. BLAZE-1, a Phase 2 clinical study of newly diagnosed participants with COVID-19 (NCT04427501) is underway. Based on actual patterns, enrolment is expected to be completed in September, with initial data readout shortly afterwards accompanied by complete data in Q4.
LY-CoV555 has been well tolerated at all doses studied and no drug-related significant adverse effects (SAEs) have been identified to date. Efficacy data is not yet available.
Lilly is dedicated to the fast release of significant scientific data. Large-scale production of this potential therapy continues, with the aim of having more than one hundred thousand doses available by the end of the year, should LY-CoV555 prove to be a substantive therapeutic option for COVID-19.
About Lilly’s COVID-19 Efforts
Eli Lilly, recognised as one of the largest pharmaceutical companies in the world, brings a full spectrum of scientific and medical expertise to aid the global response to the coronavirus pandemic. Established Lilly drugs are now being analyzed to clarify their role in the treatment of complications of COVID-19, and the firm is partnering with two biotech companies to develop new antibody therapies for COVID-19.
Lilly plans to study both single antibody treatment and antibody blends (sometimes known as antibody cocktails) as possible therapies for COVID-19.
About Eli Lilly and Company
Lilly is a public health pioneer who blends treatment with innovation to develop drugs that help the suffering people around the globe. The leading big pharma company was established more than a century ago by a man dedicated to developing high-quality medicines that serve real needs. Today, in all its work, the company remains true to that purpose.
Across the world, Lilly employees are striving to find and introduce life-changing drugs to people in need, to enhance awareness and disease management, and to give back to societies through philanthropy and volunteerism.
For more information, please contact Lilly at www.lilly.com and www.lilly.com/news