Inovio Pharmaceuticals Stock up 16.36% as HPV drug shows promise
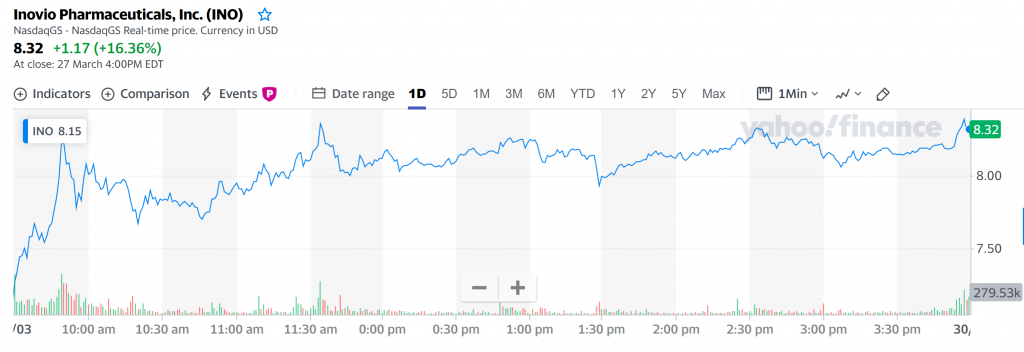
Inovio Pharmaceuticals stock soared by +16.36% on Friday, as HPV drug VGX-3100 showed promise in Phase 2 Trial.
Shares of Inovio Pharmaceuticals (NASDAQ:INO), a biotech company focusing on developing DNA medicines to treat cancers and infectious diseases, gained +16.36% at the close of trading on Friday (27 March 4:00PM EDT).
Inovio Pharmaceuticals VGX-3100 shows success in treating anal dysplasia
The leap came following Inovio’s press release on positive interim results on Thursday from a Phase 2 clinical trial evaluating the safety and efficacy of experimental drug VGX-3100 in treating patients with anal dysplasia. The disease is also known as high grade squamous intraepithelial lesion (HSIL) and is a precancerous condition caused by human papillomavirus (HPV types 16/18).
VGX-3100 to treat multiple HPV associated diseases
Ten out of twenty subjects (50%) in the clinical trial showed clearance of precancerous lesions associated with HPV types 16/18. Fifteen patients (75%) had an overall decrease in the number of lesions 6 months after beginning treatment with Inovio’s lead DNA medicine candidate VGX-3100. No cases of anal cancer have been observed in the trial. Notably, the results support the ability of VGX-3100 to treat multiple HPV associated diseases.
The sharp rise in stock value may be down to investors seeing the results of the VGX-3100 Phase 2 study for anal dysplasia as a promissing sign for the medication’s chances in a late-stage study targeting cervical dysplasia. The trial findings are also potentially promising for another Phase 2 study of the drug in treating HPV-related vulvar dysplasia.
In the pipeline
But, there are other important developments that make the Inovio Pharmaceuticals share price one to watch for investors. Inovio expects to report top-line data from its Reveal 1 late-stage study evaluating VGX-3100 in treating cervical dysplasia by Q4 of 2020. The U.S. biotech is also developing an experimental vaccine for protection against the new coronavirus disease COVID-19. Inovio hopes to begin clinical testing of the vaccine in April.