Janssen’s Golimumab gets FDA nod for pediatric use in Juvenile Arthritis
Janssen’s Golimumab, a human monoclonal antibody used as an immunosuppressive drug and sold under the brand name Simponi, has received the FDA’s approval for pediatric use in active polyarticular juvenile idiopathic arthritis (pJIA) and active psoriatic arthritis (PsA).
The Janssen Pharmaceutical Companies of Johnson & Johnson announced on 30th Sept. that the U.S. Food and Drug Administration (FDA) has approved SIMPONI ARIA® (golimumab) for patients two years of age and older for the treatment of active pJIA and has extended the PsA indication for this same patient population.
SIMPONI ARIA® (Golimumab) first and only human agent approved for pJIA and PsA
Administered by intravenous (IV) infusion, SIMPONI ARIA is the first and only fully human anti-tumor necrosis factor (TNF)-alpha biologic agent, approved for pediatric use in both active polyarticular juvenile idiopathic arthritis (pJIA) and active psoriatic arthritis (PsA).
Phase 3 GO-VIVA clinical trial adds to the growing body of evidence for SIMPONI ARIA.
Mathai Mammen, M.D., Ph.D., Global Head, Janssen Research & Development, Johnson & Johnson stated:
“This latest FDA approval of SIMPONI ARIA for pediatric use in active pJIA and active PsA not only brings a new option to young patients living with these diseases but also adds to the growing body of evidence for this treatment. For more than 20 years, we at Janssen have been committed to researching anti-TNF biologic agents for immune-mediated diseases and are encouraged to expand treatment options for these patients.”
About polyarticular juvenile idiopathic arthritis (pJIA) and active psoriatic arthritis (PsA)
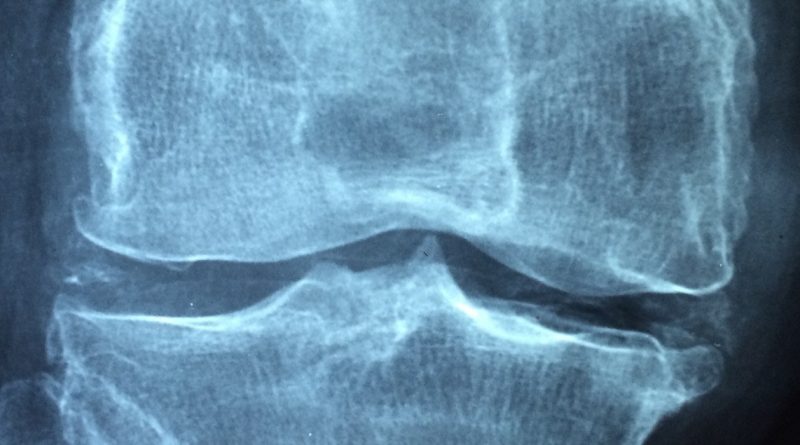
Juvenile idiopathic arthritis (JIA) is the most common type of arthritis in kids and teens and is a category of diseases defined by arthritis that occurs for at least six weeks before the age of 16. About 300,000 children in the United States suffer from any form of JIA. The most common polyarticular form of JIA is inflammation in more than four joints and resembles adult rheumatoid arthritis (RA).
PsA is one of the rarest subtypes of JIA in pediatric patients and is distinguished by both joint inflammation and psoriasis-associated skin lesions resembling adult PsA. Considering the heterogeneity of these diseases, these cases require a specific therapeutic choice.
Seth D. Ginsberg, Co-Founder and President of the Global Healthy Living Foundation and CreakyJoints commented:
“For far too long, children with pJIA or PsA have had limited treatment options. This approval represents an important step forward for these children and their families.”
Data Supporting the Golimumab Approval
The approval was based on scientific evidence from the GO-VIVA Phase 3 clinical trial results. The GO-VIVA Phase 3 clinical study was an open-label study in children ages 2 to 17 years with JIA who had active polio arthritis in five or more joints, despite receiving treatment with methotrexate for at least two months.
The findings of the trial revealed that pharmacokinetic (PK) response to SIMPONI ARIA was consistent with that of two pivotal SIMPONI ARIA Phase 3 clinical trials in adult patients with moderately to severely active RA and active PsA.
During Week 52, effectiveness was evaluated as positive endpoints. The effectiveness was generally compatible with the responses of RA patients. The adverse reactions reported in GO-VIVA were consistent with the established safety profile for SIMPONI ARIA in adult patients with RA and PsA.
Daniel J. Lovell, the Joseph E. Levinson Professor of Pediatric Rheumatology at Cincinnati Children’s Hospital Medical Center said:
“Due to the limited availability of pediatric patients for inclusion in clinical trials, it can be challenging to build clinical studies for this young patient population. Given these challenges, I am pleased to see Janssen advance the approval of a new treatment option for pediatric patients with pJIA and PsA – an important milestone in the treatment of these complex, heterogeneous diseases.”
About the GO-VIVA Clinical Trial
GO-VIVA is a Phase 3, open-label, single-arm, multicenter trial performed in nine countries with the novel therapy administered to 127 JIA patients with active polyarthritis despite currently receiving treatment with methotrexate.
Under the Pediatric Study Equity Act (PREA), GO-VIVA was undertaken as a post-marketing requirement following the initial approval of SIMPONI ARIA for adults with moderately to severely active RA in 2013.
GO-VIVA was designed to assess the dosing of SIMPONI ARIA in children that are required to achieve drug levels and response proven to be safe and effective in adults with moderately to severely active RA, since active pJIA, active PsA in pediatric patients, and adult RA show similarities in disease.
About SIMPONI ARIA (golimumab)
SIMPONI ARIA is approved in the U.S. for the treatment of adults with moderately to severely active RA, active PsA and active ankylosing spondylitis (AS), in addition to the treatment of children aged 2 years and older with active pJIA or active PsA. SIMPONI ARIA is officially licensed for one or more of these indications in 24 countries. SIMPONI ARIA is a fully human anti-TNF-alpha monoclonal antibody that targets both soluble and transmembrane bioactive forms of human TNF-alpha, a protein that when overproduced in the body due to chronic inflammatory diseases can cause inflammation.
The SIMPONI ARIA weight-based dose regimen for adults with RA, PsA, and AS is 2 mg/kg given as an IV infusion over 30 minutes at weeks 0 and 4, and every 8 weeks afterwards, respectively. In order to treat RA, SIMPONI ARIA is combined with methotrexate. The dose regimen standardized on SIMPONI ARIA body surface area (BSA) for pediatric patients with pJIA and PsA is 80 mg / m2 given as an IV infusion over 30 minutes at weeks 0 and 4, and every 8 weeks thereafter.
For more information please visit: www.janssen.com
Related Companies
More headlines