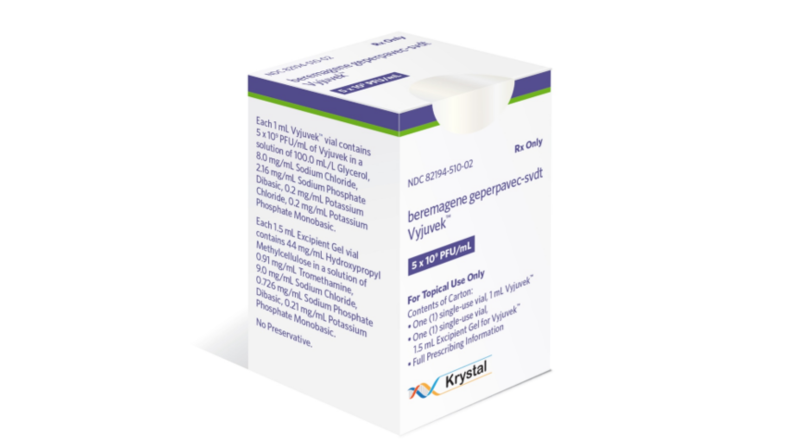
FDA Greenlights Iovance’s AMTAGVI™ as First T-cell Therapy for Solid Tumor Cancer
Iovance Biotherapeutics, Inc., a leading biotechnology firm specialising in pioneering, developing, and delivering advanced polyclonal tumor infiltrating lymphocyte (TIL) cell
Read more