FDA approves Lynparza as maintenance treatment with bevacizumab for HRD-positive ovarian cancer
AstraZeneca and Merck & Co have received the FDA’s approval for Lynparza (olaparib) as 1st-line maintenance treatment with bevacizumab for HRD-positive advanced ovarian cancer.
Lynparza (olaparib) in synergy with bevacizumab authorised in the U.S.
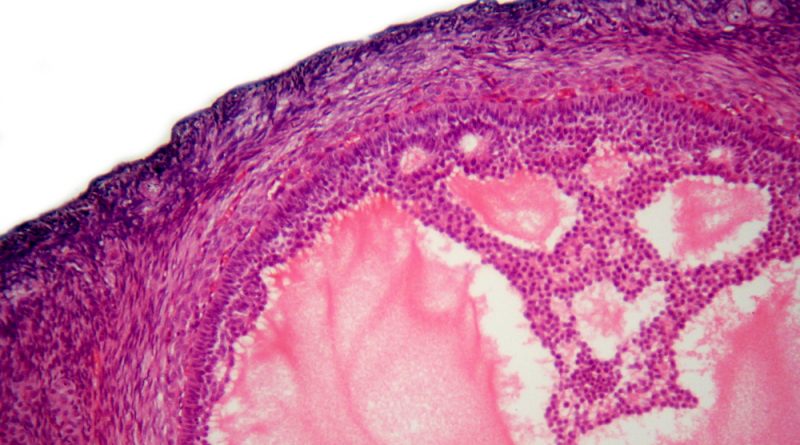
AstraZeneca and MSD Inc., Kenilworth, N.J., US (MSD: known as Merck & Co., Inc. inside the U.S. and Canada) declared on May 11, that Lynparza (olaparib) in synergy with bevacizumab has been authorised in the United States for the maintenance treatment of adult patients with advanced epithelial ovarian, fallopian tube or primary peritoneal cancer following absolute or partial response to 1st-line platinum-based chemotherapy and whose cancer is linked with homologous recombination deficiency (HRD) positive status defined by either a detrimental or potentially deleterious BRCA mutation and/or genomic instability. Patients will be selected for treatment on the basis of a companion diagnostic test approved by the US Food and Drug Administration (FDA).
Following this approval AstraZeneca will receive $100m MSD Inc. in collaboration revenue, anticipated to be booked by the company during the second quarter of 2020.
Lynparza with bevacizumab decreased risk of death by 67%
In the US, bevacizumab was licensed for use in 2018 for the 1st-line treatment of advanced ovarian cancer in conjunction with chemotherapy. Nearly half of all women with advanced ovarian cancer undergo this combined therapy within two years. The implementation of Lynparza escalated the progression-free survival (PFS) in patients with HRD-positive advanced ovarian cancer to a median of 37.2 months compared to 17.7 months with bevacizumab alone.
Suppressing progression of ovarian cancer
The FDA authorisation was based on a Phase III PAOLA-1 biomarker subgroup which revealed that Lynparza, in conjunction with bevacizumab maintenance therapy, decreased the risk of disease development or death by 67 percent (equal to a hazard ratio of 0.33).
Roy Baynes, Senior Vice President and Head of Global Clinical Development, Chief Medical Officer, MSD Research Laboratories, said:
“Advances in understanding the role of biomarkers and PARP inhibition have fundamentally changed how physicians treat this aggressive type of cancer. Today’s approval based on the PAOLA-1 trial highlights the importance of HRD testing at diagnosis to identify those who may benefit from Lynparza in combination with bevacizumab as a 1st-line maintenance treatment.”
Ovarian cancer is the eighth most common cause of death from cancer in women worldwide. Nearly 300,000 new patients were diagnosed and almost 185,000 deaths were registered in 2018. Most women are confirmed with advanced ovarian cancer (Stage III or IV) and have a 5-year survival rate of around 30 percent. Around 50 percent of ovarian cancers are HRD-positive including BRCA1/2 mutation and approximately 22 percent of ovarian cancers have BRCA1/2 mutation.
The primary goal of 1st-line treatment for patients with advanced ovarian cancer is to suspend the progression of the disease for as long as possible with the target of generating long term remission.
The double-blind Phase III clinical trial
Isabelle Ray-Coquard, principal investigator of the PAOLA-1 trial and medical oncologist, Centre Léon Bérard and President of the GINECO group, said:
“Ovarian cancer is a devastating disease. The magnitude of benefit in HRD-positive patients in the PAOLA-1 trial is impactful. The combination of Lynparza and bevacizumab now provides women with HRD-positive advanced ovarian cancer with a new standard of care and I look forward to seeing this translate into clinical practice.”
PAOLA-1 is a double-blind Phase III trial testing the efficacy and safety of Lynparza in combination with bevacizumab vs. bevacizumab alone, as a 1st-line maintenance treatment for newly diagnosed advanced FIGO Stage III-IV high-grade serous or endometroid ovarian, fallopian tube, or peritoneal cancer patients who had a complete or partial response to 1st-line treatment with platinum-based chemotherapy and bevacizumab. AstraZeneca and MSD announced in August 2019 that the trial met its primary endpoint of PFS. The full results from the Phase III PAOLA-1 trial were published in The New England Journal of Medicine.
Simultaneously, the Myriad Genetics myChoice CDx test has been approved in the US as a companion diagnostic for Lynparza in this new indication.
Homologous recombination deficiency (HRD)
HRD, which defines a sub-group of ovarian cancer, includes a wide variety of genetic abnormalities, including BRCA mutations. As with BRCA gene mutations, HRD interferes with normal cell DNA repair mechanisms and confers sensitivity to PARP inhibitors including Lynparza.
Lynparza (olaparib) is a first-in-class PARP inhibitor
Lynparza (olaparib) is a first-in-class PARP inhibitor and the first targeted treatment to block DNA damage response (DDR) in cells/tumours harbouring a deficiency in homologous recombination repair, such as mutations in BRCA1 and/or BRCA2. Inhibition of PARP with Lynparza enables the trapping of PARP bound to DNA single-strand breaks, stalling of replication forks, their collapse and the generation of DNA double-strand breaks and cancer cell death. Lynparza is being tested in a range of PARP-dependent tumour types with defects and dependencies in the DDR pathway.
Regulatory Approvals
Lynparza is authorised presently for the maintenance treatment of platinum-sensitive relapsed ovarian cancer in a variety of countries including those in the EU. It is approved as a 1st-line maintenance medication for BRCA-mutated advanced ovarian cancer upon response to platinum-based chemotherapy in the US, the EU, Japan, China and many other countries. It is also licensed in the US, Japan, and many other countries for BRCA-mutated germline, HER2-negative, metastatic breast cancer, traditionally treated with chemotherapy.
Lynparza is accredited for the treatment of germline BRCA-mutated metastatic pancreatic cancer in the US and a number of other countries. Regulatory examinations for reproductive, breast, pancreatic, and prostate cancers are underway in many jurisdictions.
Lynparza, was engineered and commercialised by AstraZeneca and MSD, and has been used for the treatment of over 30,000 patients globally. Lynparza has the most comprehensive and advanced clinical study programme compared to any PARP inhibitor clinical trials. Lynparza is the backbone of AstraZeneca’s industry-leading portfolio of potential new treatment configuration targeting DDR mechanism in cancer cells.
The AstraZeneca and MSD strategic oncology collaboration
In July 2017, AstraZeneca and Merck & Co., Inc., Kenilworth, NJ, USA, known as MSD outside the US and Canada, unveiled a global strategic oncology partnership to co-develop and co-commercialize Lynparza, the world’s first PARP inhibitor, and Koselugo (selumetinib), a MEK multiple cancer types inhibitor.
The companies agreed to develop Lynparza and Koselugo in partnership as monotherapies, as well as in conjunction with other potential new medicines. Moreover, Lynparza and Koselugo are developed by the companies in synergy with their respective PD-L1 and PD-1 medicines.
AstraZeneca in oncology
AstraZeneca has a rich oncology legacy and delivers an increasingly growing pipeline of innovative drugs which have the capacity to transform both, patients lives and the company’s forthcoming perspective. With six novel drugs introduced between 2014 and 2020 and a wide range of small molecules and biologics, in the pipeline, the organization is dedicated to promoting oncology as a major growth engine for AstraZeneca centered on lung, ovarian, breast and blood cancer.
In parallel to AstraZeneca’s core capacities, the company is constantly exploring new collaborations and acquisitions that boost the performance of its strategy, as demonstrated by the investment in haematology at Acerta Pharma. Through integrating the strength of four scientific technologies – Immuno-Oncology, Tumor Drivers and Resistance, DNA Damage Response and Antibody Drug Conjugates – and pioneering the creation of customised therapies, AstraZeneca has a vision for transforming cancer therapy and, one day, eradicating cancer as a cause of death.
More about AstraZeneca
AstraZeneca is a science-led multinational biopharmaceutical organisation focused on the research, development and marketing of prescription drugs, mainly for the treatment of diseases in three therapeutic fields – Oncology, Cardiovascular, Renal & Metabolism, and Respiratory & Immunology. The Cambridge based company is trading in over 100 countries and millions of patients globally are treated with its groundbreaking drugs.
For more information please visit: astrazeneca.com
Advertising