UK approves Pfizer and BioNTech’s COVID-19 vaccine for emergency use
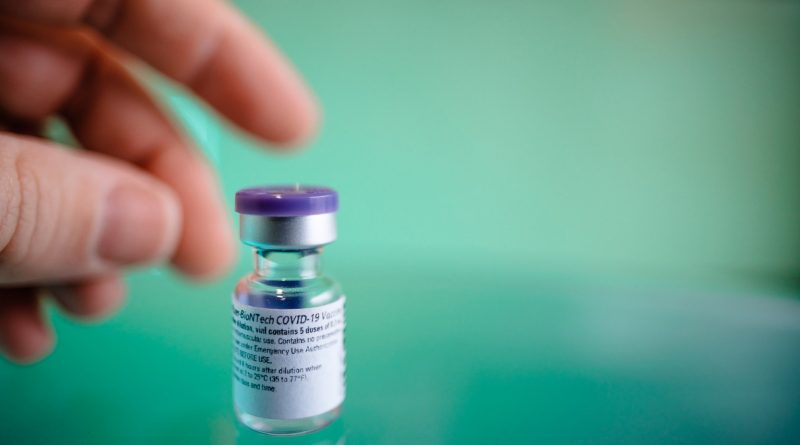
The UK has become the first western country to approve Pfizer and BioNTech’s COVID-19 vaccine for emergency use. The United Kingdom Medicines & Healthcare Products Regulatory Agency has authorised Pfizer and BioNTech’s COVID-19 mRNA vaccine for emergency supply, paving the way for mass vaccination.
UK becomes the first country to approve Pfizer and BioNTech’s COVID-19 vaccine
Pfizer and BioNTech announced today that the MHRA in the U.K. has granted a temporary authorization for emergency use for their COVID-19 mRNA vaccine (BNT162b2). This is the first Emergency Use Authorisation to be approved following a global Phase 3 trial of a vaccine to combat and end the COVD-19 pandemic.
The vaccine distribution to the designated points in the UK will be performed in compliance with the priority list, reflecting the most vulnerable groups of the population, according to the Joint Committee on Vaccination and Immunisation guidelines (JCVI).
The UK Prime Minister, Boris Johnson welcomed the news, tweeting:
In the coming days and weeks, Pfizer and BioNTech expect more regulatory actions across the globe and are in a state of readiness to supply the needed vaccine doses following future regulatory authorisations or approvals. Pfizer and BioNTech have already applied for Emergency Use Authorization with the U.S. Food and Drug Administration (FDA) and have submitted the final Conditional Marketing Authorization Application (CA) following rolling submissions with the European Medicines Agency (EMA) and several other regulatory agencies around the world.
Albert Bourla, Chairman and Chief Executive Officer of Pfizer stated:
“Today’s Emergency Use Authorization in the U.K. marks a historic moment in the fight against COVID-19. This authorization is a goal we have been working toward since we first declared that science will win, and we applaud the MHRA for their ability to conduct a careful assessment and take timely action to help protect the people of the U.K.. As we anticipate further authorizations and approvals, we are focused on moving with the same level of urgency to safely supply a high-quality vaccine around the world. With thousands of people becoming infected, every day matters in the collective race to end this devastating pandemic.”
Ugur Sahin, M.D., CEO and Co-founder of BioNTech added:
“The Emergency Use Authorization in the U.K. will mark the first time citizens outside of the trials will have the opportunity to be immunized against COVID-19. We believe that the roll-out of the vaccination program in the U.K. will reduce the number of people in the high-risk population being hospitalized. Our aim is to bring a safe and effective vaccine upon approval to the people who need it. The data submitted to regulatory agencies around the world are the result of a scientifically rigorous and highly ethical research and development program.”
First doses to be delivered to the UK within days
Pfizer and BioNTech signed a contract with the U.K. in July 2020 to provide 30 million doses of their BNT162b2 mRNA-based COVID-19 vaccine once approved for emergency use. In early October, the deal was expanded to 40 million doses which will be distributed in sections across 2020 and 2021 to ensure an equal and fair distribution of vaccine doses to the designation points with executed contracts. The first doses are expected to be delivered in the U.K. in the coming days.
The Phase 3 Clinical Trial
The UK’s MHRA temporary authorisation for emergency use was granted following continuous comprehensive data collection, monitoring and analyses including the data from a Phase 3 clinical study results confirming 95% (p<0.0001) vaccine efficacy, in participants without prior SARS-CoV-2 infection (first primary objective) and also in participants with and without prior SARS-CoV-2 infection (second primary objective), in each case measured from 7 days after the second dose.
As defined in the research protocol, the first primary objective analysis is based on 170 COVID-19 cases. To date, the efficacy was consistent among demographics of age, gender, race and ethnicity, with more than 94% effectiveness reported in adults 65 years and older. BNT162b2 was typically well tolerated in the study, with no serious safety issues observed to date by the Data Management Committee. Today’s decision is also based on a study of the Chemistry, Manufacturing and Control (CM) of Pfizer and BioNTech.
UK’s vaccine doses will be manufactured at Pfizer’s site in Belgium
Despite the enormous logistical challenges, the companies remain confident in their capability to deliver the vaccine to the people in the U.K. safely and efficiently. The joint production network of Pfizer and BioNTech has the ability, based on existing estimates, to supply up to 50 million vaccine doses globally in 2020 and up to 1.3 billion doses by the end of 2021 (subject to manufacturing capacity and regulatory approval or authorisation).
Critical to distribution in the U.K. will be Pfizer’s manufacturing site in Puurs, Belgium, one of Pfizer’s largest sterile injectable sites. The Puurs site is being used primarily for European supply but will also serve as back up supply to Kalamazoo, Michigan, for the U.S. market.
Pfizer and BioNTech’s COVID-19 vaccine distribution challenge is particularly complex as the vaccine must be stored at a temperature of -70C (-94F). But Pfizer has vast experience and expertise in cold-chain shipping and has an established infrastructure to supply the vaccine worldwide, including distribution hubs that can store vaccine doses for up to six months.
The ultra cold storage supply chain challenge
Pfizer’s distribution is focused on a versatile just-in-time method that can easily ship the frozen vials to specified points of vaccination at the moment of need. This will minimize the need for long-term storage anywhere. In a pandemic scenario, such as the current COVID-19 pandemic, vaccines are supposed to be fast to satisfy the high demand and Pfizer does not anticipate that the vaccine will need to be stored at any location for more than 30 days.
Pfizer and BioNTech have built specially constructed, temperature-controlled shippers for the BNT162b2 vaccine to ensure product safety, and can sustain recommended storage conditions (-70 °C ±10 °C) without any external equipment except dry ice for extended periods of time. Unopened, the shipper will sustain temperatures for 10 days, enabling transportation to markets worldwide.
The specially designed shippers can be used safely as a temporary storage alternative in vaccination centres to ensure the maintenance of the recommended storage temperature conditions (-70°C ±10°C) up to 30 days with re-icing every five days in compliance with the handling guidelines. Each shipper is equipped with a GPS-enabled thermal sensor monitoring 24/7 the location and temperature of each vaccine shipment. When thawed, it is safe to store the vaccine vial in a refrigerated (2-8 °C) state for up to five days.
COVID-19 vaccine manufacturing and distribution capabilities
Pfizer and BioNTech devoted efforts in collaboration with governments and Health Ministries globally to distribute the vaccine fairly and equally in accordance with the countries authorisation or approval, to make it accessible to those who need it globally. The companies are using top vaccine production and logistics capacities to rapidly produce and deliver huge volumes of high-quality vaccines, complementing BioNTech’s mRNA manufacturing experience built over nearly a decade.
Pfizer has a 171-year track record in discovering, producing, distributing and supplying people in need with groundbreaking medicines and vaccines.
BioNTech is able to manufacture mRNA for commercial supply from its current mRNA manufacturing sites in Mainz and Idar-Oberstein, Germany, after having already provided candidate doses of the vaccine for clinical trials. BioNTech will further expand its production capacity in 2021, once a third production site in Germany starts manufacturing the additional capacity for a global supply of the potential vaccines.
Recommended Companies
Ad
More Headlines