Metabolites & Impurities for Pharmaceutical Research
Expert Synthesis Solutions (ESS) is a globally trusted partner for pharmaceutical R&D professionals offering a unique catalog of high-purity metabolites, process-related impurities, and degradation compounds. With a portfolio of over 150 speciality metabolites and impurities, including many exclusively manufactured and rare compounds, ESS enables advanced drug development and bioanalytical testing with unparalleled accuracy and reliability.
From chiral intermediates to stability-testing standards, all ESS compounds are synthesized in-house, empowering researchers to achieve precision in pharmacokinetic studies, impurity profiling, and quality control workflows.
Metabolites and Impurities for Pharmaceutical Research
ESS’s metabolites and impurities portfolio addresses critical needs in pharmaceutical research:
- Drug Metabolites – Key for ADME (Absorption, Distribution, Metabolism, Excretion) studies and toxicity assessments.
- Process-Related Impurities – Identified and quantified to meet ICH guidelines for drug safety.
- Degradation Products – Critical for stability testing and shelf-life determination.
- Stereoisomers & Enantiomers – High-purity standards for chiral separations and bioequivalence studies.
These compounds are indispensable for drug metabolism and pharmacokinetics (DMPK), impurity profiling, and regulatory submissions.
Highest Quality Metabolites and Impurities for R&D Applications
All ESS compounds are classified as R&D-grade and synthesized to prioritize structural accuracy, isotopic purity (where applicable), and batch-to-batch reproducibility. While ESS is not ISO certified, each metabolite and impurity undergoes in-house quality control protocols to ensure reliability. Advanced analytical techniques such as HPLC (for purity verification), NMR spectroscopy (structural confirmation), and mass spectrometry (molecular weight validation) are employed to validate every compound.
A Certificate of Analysis (CoA) is available for each in-stock product, detailing purity levels, analytical methods, and spectral data (e.g., NMR, MS). Researchers can review and download CoAs directly from the ESS web store prior to purchase, ensuring full transparency and informed decision-making.
Key Applications in Pharmaceutical R&D
- Bioanalytical Method Development: Metabolite quantification in plasma, urine, and tissue homogenates using LC-MS/MS.
- Forced Degradation Studies: Identification of degradation pathways for ICH-compliant stability protocols.
- Chiral Separations: Resolution of enantiomers for pharmacokinetic profiling (e.g., Warfarin isomers).
- Impurity Mapping: Structural elucidation of synthetic by-products to meet regulatory filing requirements.
Featured Metabolites & Impurities
The company’s metabolites and impurities catalog includes a diverse selection of high-purity products, including:
Drug Metabolites
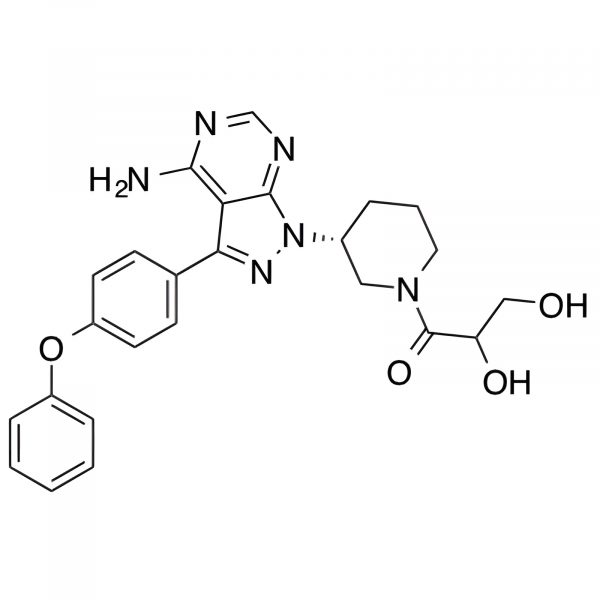
CAT#: ESS0344
CAS #: 1226872-27-0, 1654820-87-7
Purity: 98.8% by HPLC
MF: C25H26N6O4 MW: 474.51
Inventory status: IN STOCK
Application: Used to study CYP450-mediated metabolism in oncology drug development via LC-MS/MS plasma assays.

CAT#: ESS0202
CAS#: 186204-33-1
Purity: 98.6% by HPLC
MF: C28H36N4O3S MW: 508.68
Inventory status: IN STOCK
Application: Quantifies hydroxylated metabolites in antipsychotic drug permeability and CNS exposure studies.

CAT#: ESS0243
CAS#: 103877-02-7
Purity: 98.1% HPLC
MF: C16H17N3O2S MW: 315.39
Inventory status: IN STOCK
Application: A key metabolite in omeprazole research, used to study sulfide derivatives in gastric acid suppression mechanisms.
Impurities

CAT#: ESS0466
CAS#: 1000370-27-3 (free base)
Purity: 99.7% by HPLC
MF: C25H28F2N2O5S MW: 506.56
Inventory status: IN STOCK Application: Validates impurity thresholds in Parkinson’s disease therapeutics for ICH compliance.

CAT#: ESS0474
CAS#: 86718-76-5
Purity: 99.8% by HPLC (sum of axial and equatorial isomers)
MF: C24H32ClFIN3O4 MW: 607.88
Inventory status: IN STOCK Application: Essential for stability-indicating methods in gastrointestinal drug development.

CAT#: ESS0232
CAS#: 80478-42-8
Purity: 100% by HPLC
MF: C13H31Cl2NO MW: 278.22
Inventory status: IN STOCK Application: A process-related impurity for antidepressant formulations, used in forced degradation studies.
Degradation Compounds

CAT#: ESS0312
CAS#: 185107-79-3
Purity: 96.2% by HPLC
MF: C51H81NO14 MW: 932.19
Inventory status: IN STOCK
Application: A major metabolite of sirolimus (rapamycin), used in immunosuppressive therapy research and LC-MS/MS quantification in plasma for therapeutic drug monitoring and metabolic pathway studies.
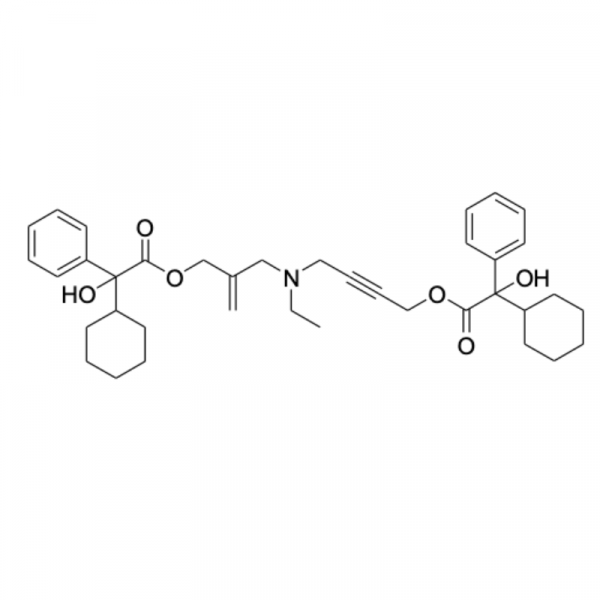
CAT#: ESS0515
CAS#: NA
Purity: 98.6% by HPLC
MF: C38H49NO6 MW: 615.80
Inventory status: IN STOCK
Application: A process-related impurity in overactive bladder therapeutics, used in stability-indicating method validation and forced degradation studies to ensure drug formulation compliance.

CAT#: ESS0320
CAS#: 7441-32-9
Purity: 99.3% by HPLC
MF: C30H24O12 MW: 576.51
Inventory status: IN STOCK
Application: A polyester degradation product, employed in analytical testing for polymer-based drug delivery systems to validate impurity thresholds and material stability under stress conditions.
Stereochemical Standards

CAT#: ESS0349
CAS#: 5543-57-7
Purity: 100% by HPLC; 99.9% ee
MF: C19H18O4 MW: 308.33
Inventory status: IN STOCK Application: A chiral reference standard for anticoagulant drug development, enabling precise resolution of enantiomers in pharmacokinetic studies and bioequivalence testing.

CAT#: ESS0348
CAS#: 5543-58-8
Purity: 100% by HPLC; 100% ee
MF: C19H16O4 MW: 308.33
Inventory status: IN STOCK
Application: The enantiomeric counterpart to S-(-)-Warfarin, used in anticoagulant research and enantiomer resolution for bioequivalence studies.

CAT#: ESS0233
CAS#: 80478-43-9
Purity: 99.9% by HPLC
MF: C13H21Cl2NO MW: 278.22
Application: A diastereomeric impurity of bupropion, critical for studying metabolic pathways and chiral separations in antidepressant formulations.
Cost-Effective Metabolites and Impurities with Global Availability
ESS provides highly cost-effective R&D-grade metabolites and impurities, delivering exceptional quality and reliability at competitive prices. The full inventory of in-stock compounds is available for immediate purchase through the ESS online store.
By eliminating ISO-certification overhead costs while maintaining the highest purity and performance standards, ESS is a stand-out partner particularly when using compounds strictly for R&D applications. As every in-stock compound is supplied with a CofA containing detailed analytical data, clients can opt to certify compounds in-house if required. In-stock items ship globally via secure logistics.
By combining quality, accessibility, transparent practices, and cost-effective options, ESS equips researchers with metabolites and impurities for fast and precise workflows with compliance readiness when required.
Why Choose Expert Synthesis Solutions?

✔ High-purity R&D-grade metabolites and impurities
✔ Catalog of more than 150 metabolites and impurities
✔ Exclusively manufactured and rare metabolites and impurities
✔ All synthesized in-house for guaranteed quality and consistency
✔ Certificate of Analysis (CofA) for every in-stock product
✔ Cost-effective R&D metabolites and impurities
✔ No ISO overhead ensuring competitive pricing
✔ Easy Client-driven ISO certification options
✔ Ready-to-ship availability
✔ Global delivery via a secure supply chain
For more information and to explore the full catalog, visit ESS Metabolites & Impurities.
Expert Synthesis Solutions continues to support pharmaceutical research with precision, reliability, and affordability in metabolite and impurity standards.






