Bi-CoV® Set for COVID-19 Sampling and RNA Isolation
The Set for COVID-19 Sampling and RNA Isolation developed by Bioinova offers high sensitivity and reliability for PCR testing along with increased safety, speed, and ease of use.
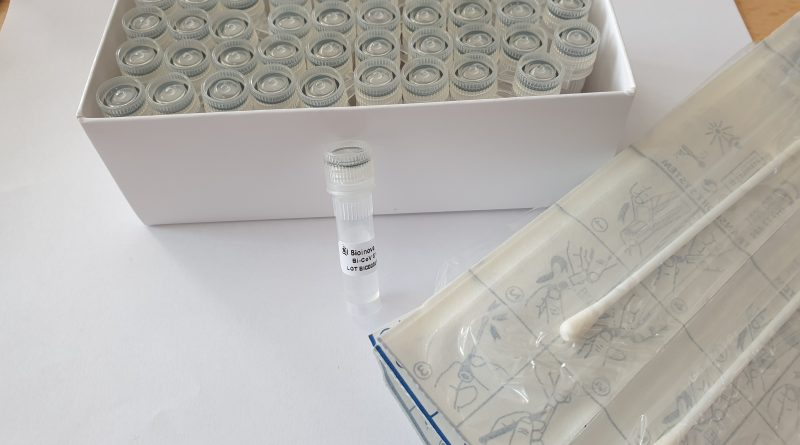
The Bi-CoV® set is a novel sample collection kit primarily used for noninvasive sampling of biological material from the oral cavity by soaking saliva into an inserted swab. It is also suitable for sample collection from other locations such as the frontal nose, nasopharynx or oropharynx.
Bi-CoV® Set for COVID-19 Sampling and RNA Isolation
The innovative kit is based on a viral transport medium that ensures a gradual decomposition of viral particles with the half-life of the viruses being approximately 40 minutes (decrease in the sample virulence by 68.4% after 1 h). Meanwhile, nucleic acid molecules, such as in the SARS-CoV-2 RNA, are spontaneously released into the solution, where they are protected against degradation for a minimum of 72 hours.
Sample Collection Kit for Fast, Reliable and Safe PCR testing
By the time of the delivery to a diagnostic laboratory, the sample is ready for PCR analysis without the need for any other chemical or physical manipulation, as the solution is compatible with the vast majority of RT-qPCR kits. If PCR analysis is desired immediately after the sample collection, it can simply be heated to 95°C for 5 minutes. During this incubation time, all viruses are quickly decomposed and the RNA is instantly ready for PCR.
Importantly, the viral inactivation ability of the solution substantially increases the safety of the personnel during sample processing.
Increase Testing Capacity through RNA Isolation Free Procedure
The RNA-isolation-free procedure saves time and reduces the cost of the analysis whilst significantly increasing the testing capacity of laboratories. Moreover, pooling of up to 10 samples has been validated with Bi-CoV®, enabling further testing time and cost reduction with no loss in sensitivity and quality of the PCR.
As for the performance, Bi-CoV® has been tested in clinical trials that revealed a 27% higher diagnostic sensitivity as compared to the standard procedure including nasopharyngeal swab, RNA isolation and PCR analysis.
Unique In vitro Diagnostic Product from Bioinova
In 2018, a new program was initiated at Bioinova – development of in vitro diagnostic methods, tools and medical devices. Two years later, the company launched its first of several planned unique in vitro diagnostic products – the Bi-CoV® set.
Since the launch in 2020, demand for Bi-CoV® has grown rapidly not only in the Czech Republic but across some countries of the European Union, Central America and South East Asia. To satisfy the high demand, Bioinova is increasing its manufacturing capacity and investing in modern technology and equipment for further automation of production.
In recent months, several companies from all around the world interested in obtaining Bi-CoV® license or in contract manufacturing for certain geographical areas have been approaching Bioinova.

Features
- Noninvasive sampling of biological material
- PCR analysis direct from sample
- Pooling of up to 10 samples
- RNA isolation-free procedure
- IVD Certified
- Also available for licencing
For more information please contact Bioinova today or read more about the product on the company’s website: