Automated Crystallization Imaging System
The Automated Crystallization Imaging System, XtalLight 200/210, is a desktop device that boasts full automation for rapid imaging of standard crystallization plates with exceptional quality.
The compact XtalLight 200/210 is a highly versatile system that combines bright light and UV imaging to swiftly and precisely identify crystallization hits. Beyond crystal hit identification, it serves a diverse range of functions, including trace and intrinsic fluorescence imaging, and the identification of aptamers and LCP crystals.
Automated Crystallization Imaging System
By harnessing the power of bright light and UV imaging, along with automated drop detection and trace fluorescence imaging, the automated crystallization imaging system XtalLight 200/210 guarantees swift and precise identification of crystallization hits. Positioned as a versatile middle ground between fully automated plate hotel solutions and traditional laboratory microscopes, it offers the convenience of automated imaging and data acquisition akin to hotel solutions, all within the compact footprint of a standard laboratory microscope.
Beyond the identification of crystal hits, this technology serves a diverse array of functions, spanning from trace and intrinsic fluorescence to identifying aptamers and LCP crystals. Through its bright and UV-light imaging function, the automated desktop imaging system facilitates the accurate localisation of protein components and differentiation between salt and protein crystals.
Its hallmark traits include swift operation, superior output fidelity, and a user-friendly interface. The XtalLight 200/210 system offers the option for a seamless upgrade to a high-performance dynamic light scattering (DLS) device, transforming into the SpectroLight 600/610. All data generated are efficiently stored and accessible through an integrated SQL database, ensuring comprehensive data management capabilities.
Rapid Identification of Crystallization Hits
Engineered for imaging standard crystallization plates, the Automated Crystallization Imaging System, XtalLight 200/210, operates at high speed without compromising quality, crucial for efficient screening and analysis in drug discovery.
For swift identification of crystallization hits, rapid and straightforward access to acquired imaging data is as critical as efficient data acquisition. The user interface of the XtalLight 200/210 is specifically engineered to serve both functions seamlessly. This enables exceptionally fast and precise assessment of potential drug candidates and their interactions with protein crystals.
Bright Light and UV-Imaging in LCP Crystallization
Bright and UV-light imaging facilitates the localization of protein contents and differentiation between salt and protein crystals.
When crystallizing membrane proteins using Lipidic Cubic Phase (LCP) crystallization, it is common to utilise polymeric or glass covering materials. These materials prove highly suitable for bright light and even intrinsic fluorescence imaging. In situ crystal identification is streamlined through UV illumination, as it enables the excitation of intrinsic protein fluorescence, enhancing crystal detection.
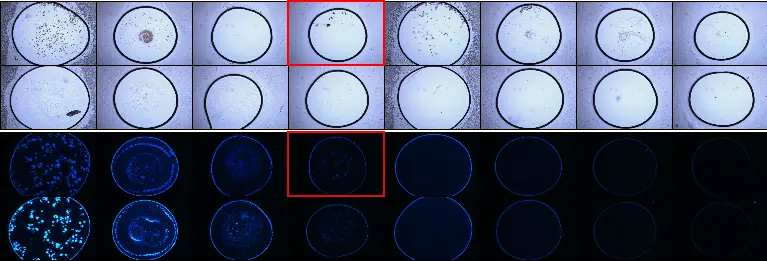
Automated Drop Finding for Identification of Small Crystals
LCP-plates, comprising 96 well plates with circular wells measuring 5 mm in diameter, typically position the LCP sample quite centered using dispensing systems. However, at higher magnifications, the LCP drop may drift out of the field of vision. Therefore, an automated drop localisation system, utilising pattern recognition, becomes essential. This feature allows for automated imaging at high magnification, facilitating the identification of even the smallest crystals.

Automated drop localisation is not restricted solely to LCP-Plates. It can be adapted to nearly all standard plate types. When paired with image focus stacking technologies, a capability of the XtalLight 210, common issues like non-centered and out-of-focus images are effectively addressed.
Intrinsic Fluorescence Imaging for Protein Crystallization
In the context of protein crystallization, intrinsic fluorescence imaging is commonly employed using UV light. This technique capitalises on the UV-fluorescence properties of tryptophan. When excited by ultraviolet light, tryptophan exhibits a distinct blue fluorescence at approximately 360 nm. The primary purpose of intrinsic fluorescence imaging is to perform an in situ assessment to determine whether a crystal is composed of salt or protein.
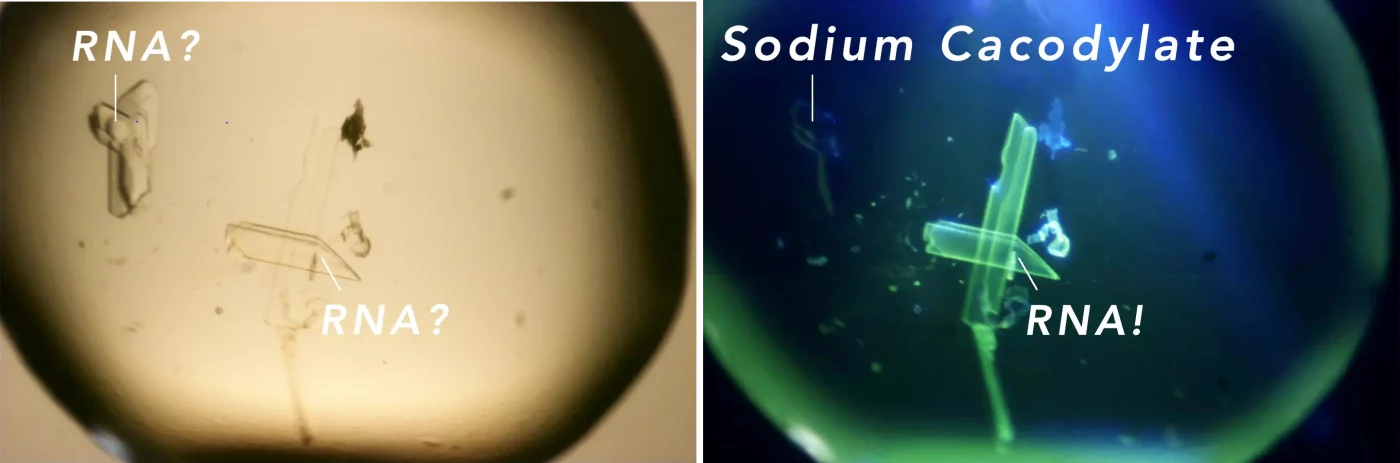
Trace Fluorescence Imaging
The trace fluorescence imaging technique relies on specific labeling of proteins with covalently attached fluorophores. Trace fluorescence is particularly valuable when the intrinsic protein fluorescence is insufficiently strong or becomes noisy, or when the camera shutter time exceeds an acceptable interval. In such cases, carboxyrhodamine is commonly employed as the fluorophore. Carboxyrhodamine offers the advantage of being excited at a maximum of 525 nm (green light), minimising radiation exposure to the crystal. Additionally, background fluorescence is often minimised as many plate materials do not fluoresce at this wavelength. Once the covalent staining protocol is established, the procedure becomes straightforward and can be routinely applied.
Advancing Pharmaceutical Research with the Automated Crystallization Imaging System – XtalLight 200/210
The XtalLight 200/210 Automated Crystallization Imaging System is a transformative asset for pharmaceutical research, enhancing drug discovery and development workflows. Its full automation and rapid imaging capabilities allow for the efficient screening of crystallization plates – vital in preclinical research where identifying potential drug candidates is time-sensitive and resource-intensive. The accurate distinction of protein crystals from salt with the XtalLight 200/210 ensures that only viable candidates move forward in the drug development pipeline, optimising resource allocation, accelerating the path to clinical trials and, ultimately, the delivery of new therapies to patients.
This method provides a minimally invasive and label-free way to verify the presence of protein, which is crucial for the accurate analysis of crystallization outcomes. Moreover, the XtalLight 200/210’s ability to handle various functions, such as identifying aptamers and nucleic acid crystals by employing nucleic acid specific non-covalent attached fluorophores, broadens its application in the pharmaceutical field. Aptamers, with their high specificity for target molecules, are key in therapeutic and diagnostic applications, whilst LCP crystals are instrumental in the study of membrane proteins, a class of proteins that are often targets for new drugs.
The compact design of the XtalLight 200/210 means it can be easily accommodated in space-constrained research environments, broadening access to advanced crystallization screening technology. This is particularly important for pharma and biotech companies and CRO’s looking to streamline their R&D operations whilst enhancing the accuracy of their findings.
XtalLight 200/210 Device Features
- Fully Automated Desktop Crystallization Imaging System
- Fast and Accurate Identification of Crystallization Hits
- Automated Drop Finding
- Bright and UV-light imaging capabilities
- Intrinsic Fluorescence Imaging
- Trace Fluorescence Imaging